ISSN : 2348-9502
American Journal of Ethnomedicine
Antimalarial Potential of Plants Used as Chewing Sticks for Oral Hygiene in Rural Areas of Rajasthan, India
Haffkine Institute for training research and Testing, Acharya Donde Marg, Parel Mumbai-400012, Maharashtra state, India
Abstract
The present study was planned to evaluate antiplasmodial activity of two plants Azadirachta indica and Acacia nilotica regularly used in primary health care and oral hygiene in rural areas of Rajasthan, India. The aim was to corroborate how traditional practices using chewing sticks (Fresh twigs of medicinal plants in place of tooth brush) in relation to control of malaria. The In vitro antimalarial activity of fresh twigs of Azadirachta Indica (neem) and Acacia nilotica (Babul) were evaluated against chloroquine sensitive Plasmodium falciparum 3D7 strain by light microscopy using Giemsa-stained smears. Azadirachta indica most commonly used in the area showed promising antiplasmodial activity with IC50 of 39.86 μg/mL as compared to Acacia nilotica the IC50 value ranged beyond 50 μg/mL (IC50 77.78 μg/mL). Both the plants studied under present investigation are known for their medicinal properties, regular use may provide a solution for better management of the disease in malaria risk areas.
Keywords
Azadirachta Indica, Acacia nilotica, Plasmodium falciparum, Antimalarial, Medicinal plants.
INTRODUCTION
Malaria is one of the most severe public health problems worldwide, though it is entirely preventable and treatable mosquito borne illness. According to the World Health Organisation’s World Malaria report 2013 and Global Malaria Action plan, 3.4 billion people (half of the world’s population) live in areas of malaria transmission in 106 countries and caused an estimated 207 million clinical episodes, and 627,000 deaths [1]. Traditional medicine occupies a central place among rural communities of developing countries for the provision of health care in the absence of an efficient public health care system. According to World Health Organization’s report, over 80% of the world population relies on traditional medicine for their primary health care [2]. For many traditional herbal medicines may be the only source of treatment available [3].
Rajasthan is well known for its biodiversity richness of diverse cultural mosaic. The state is located in the North western part of India. Geographically, it lies between 23˚3′ to 30˚12′ longitudes and 69˚30′ to 78˚17′ latitudes. Southern part of Rajasthan is the tribal belt comprising Banswara, Chittorgarh, Dungarpur, Udaipur and Rajsamand districts. Therefore, an attempt has been made during 2013-2014 to collect information about plants used by tribes and local people (aboriginal) in their traditional health care system for Rajsamand district. The plants growing around them form an integral part of their culture. The local communities use their indigenous experiences and knowledge in categorizing various medicinal plants when dealing with different ailments. The region was frequently visited with regards to collection of medicinal plants.
The cultural system tends to favor practices which minimize the risk of diseases and maximize health care4. Traditional use of plants to improve dental health and promote oral hygiene had been known since antiquity. Some of the selected plants used for such practices viz; Acacia catuchu, jatropha curcus, Thevetia peruvina, Nerium indicum, Acacia nilotica and Azadirachta indica. The subjects reported a decrease in malaria attacks using Azadirachta indica in various forms, though the diagnosis of malaria was not clinically confirmed. In the present investigation two plants Azadirachta indica and Acacia nilotica were selected for antiplasmodial activity against Plasmodium falciparum 3D7 strain.
Azadirachta indica (Meliaceae) is well known in India as one of the most versatile medicinal plants having a wide spectrum of biological activity. Every part of the tree has been used as traditional medicine for house hold remedy against various human ailments and still regarded as a village dispensary in India [5,6]. Biological and pharmacological activities that attributed to different parts of A. Indica includes antiplasmodial, anti-trypanosomal, anti-oxidant, anti-cancerous, anti-bacterial, antiviral, larvicidal and fungicidal activities [7]. The neem leaf extract reported for both schizonticidal and gametocidal activities against Plasmodium falciparum [8].
Another plant selected for antiplasmodial activity was Acacia nilotica (Family: Fabaceae and sub-family: Mimosoideae), a spiny tree native to the Indian subcontinent and most parts of Africa, tender twigs are used as tooth brush [9]. The plant is widely used in folk medicine to treat a variety of diseases with potential antioxidant activity. The gum, bark, leaves and fruits of this tree were used medicinally for cold, bronchitis, pneumonia, diarrhoea, hemorrhage, antibacterial and antiplasmodial activity [10]. Acacia nilotica had also been proved as an effective medicine in the treatment of malaria, sore throat (aerial parts) and tooth ache bark [11, 12]. Besides young stem and twigs of the neem and Babul the leaves of Papaya (Carica papaya), Tulsi (Ocimum sanctum), mint (Menthe spicata), curry leaves (Murraya koenigii) and leaf of mango (Magnifera indica) are chewed and kept in mouth for sometime as mouth freshener with remarkable medicinal properties [13]. In view of this, the present study was planned to evaluate the antimalarial activity and to correlate how traditional practices aids important inputs in control and better management of the disease.
MATERIALS AND METHODS
Plant material
Plants were collected from district Rajsamand located between latitudes 24˚46′ to 26˚01′ N and Longitudes 73˚28′ to 74˚18′ N of the state Rajasthan, India. A field survey was carried out randomly during the period January 2013- June 2014. Fresh twigs of Azadirachta indica and Acacia nilotica were collected between April-May, 2013, The plants were authenticated by Botanist Dr. Pratibha Cheturvedi, Haffkine Institute and Voucher specimen were deposited for future reference.
Preparation of extracts
The fresh twigs of both the plants were washed twice with distilled water, crushed and extracted with water using standard extraction procedures [14]. Solvents were removed under reduced pressure by using a rotary vacuum evaporator. The percent yield of the extract was determined and the crude extracts were stored at 4º C prior to antimalarial assay. Preliminary phytochemical screening was done using standard procedures [15].
Parasite cultivation
The Plasmodium falciparum 3D7 strain was procured from Indian Institute of Technology (IIT), Mumbai, India, was maintained in continuous culture by the modified method of Jensen and Trager [16] (1980) in O+ human red blood cells at a 5% hematocrite in RPMI 1640 medium, supplemented with L-glutamine (4.2mM), HEPES (25 mM), NaHCO3 (25 mM) hypoxanthine (6.8 M), 0.5% AlbumaxII (Invitrogen) and 50μg/ml Gentamicin. Cultures were incubated at 37ºC in an atmosphere of 5% CO2, 91% N2, and 3% O2. Chloroquine was used as positive control.
In vitro antiplasmodial assay
The In vitro activity was evaluated against Plasmodium falciparum by means of the Mark III test, as developed by the WHO [17]. Briefly, the parasite cultures, prior to experimentation, was synchronized to the ring stage of treatment with 5% D-sorbitol [18]. Each extract was tested in duplicate in two independent experiments. Extracts were filter sterilized and different concentrations (150, 100, 50, 25, 12.5, 6.25 and 3.125 μg/mL) were incorporated in 96 well tissue culture plate with 1-2% parasitemia and 2% hematocrite. The plates were incubated at 37º C in CO2 incubator and inhibition of parasite was determined microscopically after 48 h by using Giemsa-stained smears [19]. The control parasite culture freed from extracts was referred to as 100% growth. The IC50 values, the extract concentration required to inhibit parasite growth by 50% was determined for each extract-parasite combination.
RESULTS
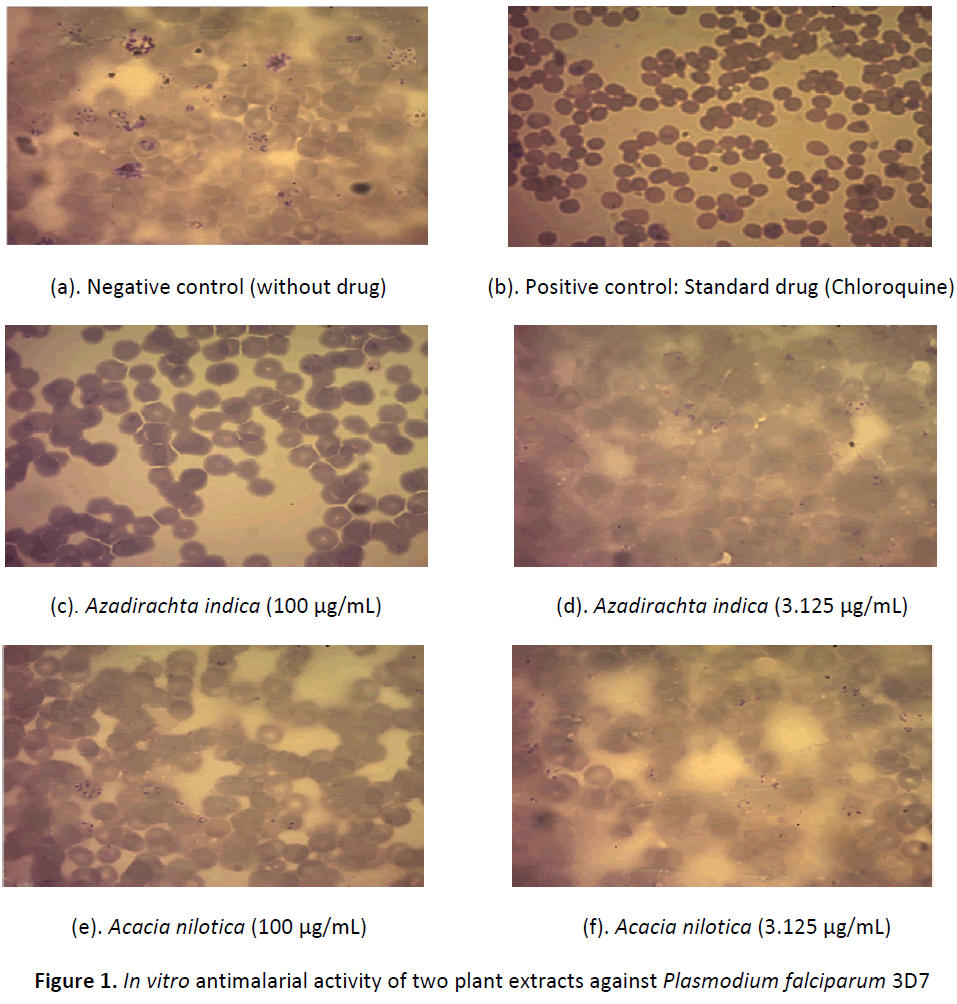
The present study investigated with fresh aqueous extracts derived from the twigs of A. indica and A. nilotica against chloroquine sensitive Plasmodium falciparum 3D7 strain by In vitro method. The results are summarized in table 1. The basic measurement of antimalarial activity used in this study was the reduction in the number of parasitized erythrocytes in extract treated test cultures as compared to control without drug at 48 h incubation period. The parasitemia decreased with increasing concentration of the extract, reflecting an inhibitory activity of parasite replications. Figure 1 (slides a-f) represents drug induced inhibition of parasitaemia. Figure 1-a represent negative control (without drug) showing very high perasitamia as compared to the positive control (chloroquine- 3.0 μg/mL) showing almost nil parasitaemia (figure 1-b). Figure 1-c and 1-d represents activity of the highest (100 μg/mL) and lowest concentration (3.125 μg/mL) of A. indica extracts. The results clearly demonstrated promising inhibitory activity of parasite replication in higher concentration as compared to lowest concentration. Figure 1-e and 1-f represents the results of A. nilotica extract showing inhibition of parasitaemia in both the concentrations, but significantly low as compared to Azadirachta indica. From the literature antimalarial activity of the extracts was defined according to the IC50 (Extract concentration that gives 50% inhibition of parasitemia) values obtained. An extract showing an IC50 value ≤ 50 μg/mL was classified as active. Extracts having activity beyond this range were considered inactive [20]. Based on this classification A. indica exhibited promising antimalarial activity with IC50 of 39.86 μg/mL as compared to Acacia nilotica the IC50 value ranged beyond 50 μg/mL (IC50 77.78 μg/mL).
Table 1: Percent inhibition of parasitemia and IC50 value of Azadirachta indica and Acacia nilotica against Plasmodium falciparum 3D7 strain
| S. No. | Plant | Percent inhibition of parasitemia Concentration of extract( μg/mL) | *IC50 ( μg/mL ) | |||||
|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | 3.13 | |||
| 1. | Azadirachta indica | 81.25 | 73.25 | 60.56 | 33.21 | 13.76 | 8.09 | 39.86 |
| 2. | Acacia nilotica | 63.56 | 32.19 | 25.01 | 11.87 | 5.32 | 2.18 | 77.78 |
*IC50 Concentration inhibiting 50% parasitemia
The preliminary phytochemical study reveals that the tested medicinal plants contain a variety of phytochemical constituents (terpenoids, alkaloids, saponins and glycocides). Each of these phytochemicals is known for various protective and therapeutic effects [21].
DISCUSSION
Traditional medicines occupy a central place among rural communities of Rajasthan. People residing in the tribal belt of Rajasthan relay significantly on local plant resources for their primary health care. A large number of plants and plant parts are used for dental and oral hygiene, according to individual preference and their contained medicinal property. Neem and Babul tree are largely populous in India and commonly grow along the roadside and around houses every few meters distance in this region. Bhasin [22] carried out a detailed survey of oral health behavior among the Bhils of Rajasthan (the third largest tribal group of India) and also reported various traditional methods and herbal remedies used by them for dental care and oral diseases. More than any other Indian herb neem proved useful in helping the body resist diseases and restore the proper balance to the body’s system [23]. The tribe and local people had a strong perception that the use of medicinal plants for dental and everyday health care keeps the body healthy and protect them from malaria fever and other common illnesses. This prompted us to test the antiplasmodial activity of twigs (chewing sticks) of two plants Azadirchta indica and Acacia nilotica, scientifically by In vitro method.
Both these plants are well known for their medicinal properties with a complex chemical makeup [24]. Regular use of these plants for a long time facilitates absorption of a part of active compounds internally. Though, the concentration of active compounds may not be enough to directly inhibit the parasites unless some other mechanisms are involved. These may include immune memory acquisition, inhibiting or inducing enzymatic processes affecting drug metabolism [25]. Sometimes the medicinal plants may act as antipyretics or may enhance the immune system, rather than having direct anti-parasitic activity [26] and thus, protecting from the severity of the disease and enhance quick recovery.
Another reason that reduces chances to get malaria may be because daily use of chewing sticks to clean the teeth with great efficiency may alter the chemical profile of the breath and that in turn reduce attraction of mosquito towards such people. Previous research reported that unique combination of chemicals that make up an individual’s breath may play a role in repelling or attracting mosquito [27]. Gametocytes in the peripheral blood attracts mosquitoes for their own survival and continuation of the lifecycle [28]. The Neem plant had been reported for its excellent gametocidal activity [7]. That may prevent mosquitoes to focus their bites on such people and thereby preventing re-infection of mosquitoes and man [29].
Regular use of medicinal plants boosts the immunity. A person with strong intrinsic immunity may get infected with malaria, but recovered quickly and such repeated infection may develop naturally acquired immunity against malaria [30]. The dual action of neem on the parasite and stimulation of the host immune system provides a major factor in the effectiveness of neem against malaria [31]. The promising antiplasmodial activity of Acacia nilotica was reported by Tahir [32] and higher analgesic activity further signifies its suitability as antimalarial agents.
The in-vitro results demonstrated antimalarial activity by direct action on the parasites. On the other hand using these plants for oral hygiene and primary health care, the mechanism of action may be on the hosts that keep the body healthy to fight the infection when it is really required [33]. Even though no toxicity test has yet been performed, the use of these plants in traditional medicine for a long time, are considered safe and non-toxic.
CONCLUSION
Current global search for plant derived natural anti-malarial mainly focused on direct anti-parasite activity and novel mode of action. However, natural compounds targeting the hosts remains a largely unexplored but an attractive area of future research for the treatment of malaria. Plants studied under present investigation provided important inputs in malaria control. Further scientific evaluation is needed to develop a practical tool for proper management of the disease in malaria risk areas.
ACKNOWLEDGEMENT
The financial support of ICMR, New Delhi (Grant code: 59/39/2010/BMS/TRM) is gratefully acknowledged.
REFERENCES
- WHO. World Malaria Report. Geneva, World Health Organization 2013.
- WHO .Traditional medicine strategy 2002- 2005. Geneva WHO 2002.
- Willcox ML and Bodekar G. Traditional herbal medicine for malaria. Brit Med J 2004; 329; 1156-1159.
- Wright CW. Traditional antimalarial and the development of noval antimalarial drugs J Ethnopharmacol 2005; 100: 67-71.
- Koul O, Isman MB, Ketkan CM. Properties and uses of Azadirachta indica. Canadian J Botany 1990; 68: 1- 11.
- Stix G. The village Pharmacy. The neem tree yields products from pesticides to soap Sci Am. 1992; 132.7.
- Ravikumar S, Jacob S, suganth LP. In vitro antiplasmodial activity of ethanolic extracts of South indian medicinal plants against Plasmodium falciparum, Asian Pac J Trop Dis 2012; 80-83.
- Udeimya J I, Shu EN, Quakyi I, Ajavi FO, 2008. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am. J. Ther 15: 108-10.
- Fagg CW. Acacia nilotica Pioneer for drug lands. Department of plant Science, University of Oxford U. K. 1990; 28-38.
- Ali A, Akhtar N, Alikhan B, 2012. Acacia nilotica: A plant of multipurpose medicinal uses with potential antioxident activity. Jour Med Plants Res 6: 1492-1496.
- Joshi P. Ethnomedicine of Trible Rajasthan- An Over view. In: Pushpangadan et al (Eds.), Gilmpses of India Ethnopharmacology, TBGRI, Thrunantha- puram, Indian: 1994; 147-162.
- Ballal AD, Bobbala SM, Qudri M, Moller D, Kompe O, Nasir A. Antimalarial effect of gum Arabic. Malaria Journal 2011; 10: 139.
- Bairwa R, Gupta P, Gupta VK, Srivastava B, 2012. Traditional medicinal plants: used in oral hygiene. Int. J. Pharma Chem Sci 2012; 1: 94.
- Kokate C K. Practical pharmacognosy Vallabh Prakashan 2000; 218.
- Harbone JB. Phytochemical methods, A guide to modern techniques of plant analysis. Chapman and Hall London 1998; 54-84.
- Jensen JB, Trager W. Cultivation of erythrocytic and exoerythrocytic stages of Plasmodium in malaria. Academic Press.1980; 2: 280.
- WHO. In vitro micro test (mark III) for the assesment of the response of plasmodium falciparum to chloroquine, mefloquine, quinine, and artemisinin. Division of control of Tropical Disease Review 2001; 2 CTD/MAL/97.20.
- Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. Journal of Parasitology 1979; 65: 418-420.
- Le Bras J, Deloron. In vitro study of drug sensitivity of Plasmodium falciparum: evaluation of a new semi-microtest. Am J of Trop Med and Hyg 1983; 274:1421–1422.
- Ramazani, A, Zakeri, S, Sardari, S, Khodakarim, N. Djadidt, ND. In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malaria Journal 2010; 9: 1-8.
- Saxena S, Pant N, Jain DC, Bhaluni RS. Antimalarial agents from plant sources. Cur Sci 2003; 85: 1314-1326.
- Bhasin V. Oral health behavior among Bhils of Rajasthan. Journal Soc Sci 2004; 8: 1-5.
- Dey AC, Indian medicinal plants used in Ayurvedic preparations Dehra Dun India; 1980: 165-166.
- Anonymous. The Wealth of India, Raw Material, Vol 1, CSIR, New Delhi, 2004, 11.
- Steven maranz. An alternative paradigram for the role of antimalarial plants in Africa. The Scientific World Journal 2012; 2012: 9.
- Phillipson, JD, Wright CW. Medicinal plants against protozoal diseases. Trans of the Royal Soc of Trop Med and Hyg 1991; 85: 155-165.
- Mukabana WR, Takken W, Killeen GF. Allomonal effect of breath contributes to differential attractiveness of human to the African malaria vector Anopheles gambiae. Malaria journal 2004; 29: 3(1), 1.
- Ronaud Lacroix, Mukabana WR. Malaria infection increases attractiveness of humans to mosquito Journal PLOS/Biology 2005.
- Bowman, W.C.; Rand, M.J. Chemotherapy of protozoan infections. In Textbook of Pharmacology; Publication Blackwell Scientific: Oxford, UK, 1980; pp. 36.1–36.5.
- Denise L Doolan Carlota Dobano J Kevin baird. Acquired immunity to malaria. Clinical Microbiol Rev 2009; 22: 13-36.
- Contrick J. Neem: The ultimate herb, Lotus Press Ist edition 2001.
- Tahir AEI, Gwira M, H Satti and Sani A Khalia. Antiplasmodial activity of selected Sudanese medicinal plants with emphesis on Acacia nilotica. Phytotherapy Research 1991; 13: 475-478.
- Benoit VF. Ethnomedicine in malaria treatment. I Drugs 2005; 8 (1):45-52.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences