ISSN : 0976-8505
Der Chemica Sinica
Anti-Inflammatory, Proton Pump Inhibitor and Synthesis of Some New Benzimidazole Derivatives
Rezk R Ayyad1,5, Helmi M Sakr1, Kamal M El-Gamal2,4*, Ibrahim H Eissa1, Ahmad HA Tita1, Adel S Abd El-Raheim1, Farag F Sherbini2 and Ahmad M Mansour3
1Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo, Egypt
2Organic Chemistry Department, Al-Azher University (Boys), Nasser City, Cairo, Egypt
3Pharmacology Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo, Egypt
4Organic Chemistry Department, Faculty of Pharmacy, Delta University for Science and Technology, Gammsa, Egypt
5Medicinal Chemistry Department, Faculty of Pharmacy, Delta University for Science and Technology, Gammsa, Egypt
Abstract
A series of novel of 1 and 2-substituted benzimidazoles was synthesized by the reaction of Phenelenediamine with p-hydroxybenzaldehyde. All the synthesized compounds were characterized by IR, 1H-NMR, 13C-NMR, and elemental analysis. The newly benzimidazoles derivatives were screened for analgesic and anti-inflammatory activities on carrageenan induced paw oedema in rats. Among all the benzimidazol derivatives, compound 8b and 10 exhibited significant analgesic and anti-inflammatory activities. Acute ulcerogenicity studies showed that compound 6 did not cause any gastric mucosal lesions in the rat stomach at the dose (300 mg/kg) indicating that this compound is devoid of gastric irritant properties.
Keywords
Benzimidazole, Anti-inflammatory, Proton pump inhibitor, Docking
Abbreviation
M.P: Melting Point; DMF: Dimethyl Formamide; TLC: Thin Layer Chromatography; DMSO: Dimethyl Sulfoxide; CDCl3: Deuterated Chloroform
Introduction
The cause of inflammation includes physical agents, chemical agents, immunological reactions, and infection by a pathogenic organism [1]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the most widely used drug categories against inflammation, mild to moderate pain and fever this is essentially brought about by inhibiting the rate limiting cyclooxygenase (COX) enzyme involved in the inflammatory cascade [2]. However, long term medication of NSAIDs is associated with adverse effects like hepatotoxicity, platelet dysfunction and bleeding [3,4]. But the major side effect is gastrointestinal (GI) ulcerations due to inhibition of cyclooxygenase (COX) in tissues exerted with chronic use of NSAIDs. Consequently, a real need exists to develop new anti-inflammatory, analgesic effect with good efficacy, fewer toxicity and less side effects of gastric ulceration. Herein we need to discovery and development of novel anti-inflammatory and analgesic agents along with safety profile is still a necessity. On synthesizing novel chemical modifications or derivatization of existing benzimidazole moieties could lead to neutral molecules with greatly reduced acidic ulceration as a useful approach to explore safer and potent anti-inflammatory and analgesic agent [5]. Among different types of NSAIDs, Benzimidazole [6] which is a structural unit of naturally occurring nucleotide, because it easily interacts with the biopolymers of living systems [7] and due to this character it responsible for its numerous biological aspects like anthelmintic [8], antifungal [9], antimicrobial [10,11], antiviral [12] and antineoplastic [13] activities. The aforesaid different pharmacological activities of benzimidazoles encouraged us to study the in-vivo analgesic and anti-inflammatory activities of some important new 1. 2 substituted benzimidazole derivatives aiming at finding new gastroprotective leads with potential anti-inflammatory, analgesic activities. In the present research work, a novel series of 1, 2-substituted benzimidazole derivatives have been synthesized and bioevaluated for their anti-inflammatory, analgesic, and allergenic activity of the resulted compounds. The non-steroidal anti-inflammatory drugs become main COX inhibitors.
Molecular modelling
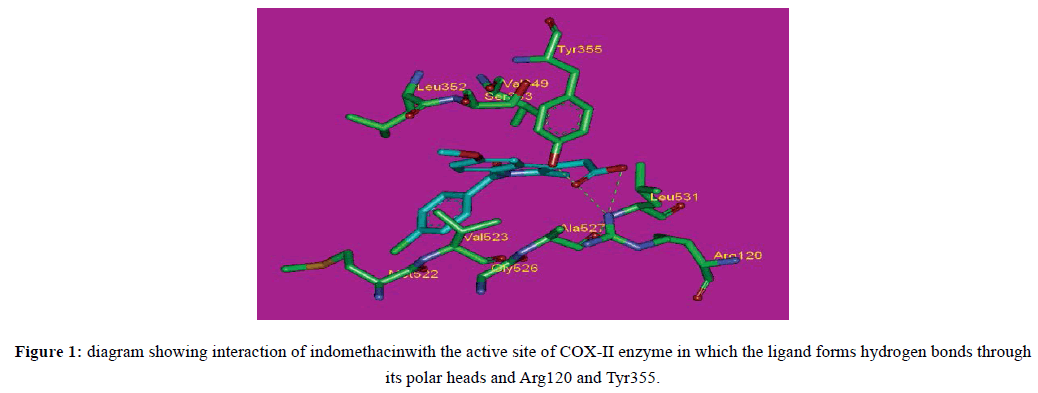
Molecular modelling is one of the most widely applied techniques in drug discovery. This branch of science is centered on applying the fundamental laws of physics and chemistry to the study of molecules. In the case of drug discovery the molecules under study are those directly or indirectly involved in human disease. The ultimate aim is to create models and simulations, which can help in the different stages of a discovery pipeline by predicting, rationalizing and estimating the properties of molecules and their interactions, thereby allowing a more rational approach to drug development [14]. Considering ligand–receptor interaction, one usually takes into account two points: strength of the interaction (what we refer to as ‘affinity’) and type of an effect at the biochemical, electrophysiological and behavioural level triggered by the ligand (what we refer to as ‘intrinsic activity). To get an insight into the type of chemical forces involved in the interaction, one either builds models of a specific pharmacophore or constructs models of a receptor protein and examines the binding forces. Interaction of a ligand with a receptor may result in receptor activation (agonists) [15]. The most popular approach to predicting the correct binding pose and binding affinity (BA) is protein-based modelling (docking) in which physicochemical interactions between a ligand and receptor are deduced from the 3D structures of both molecules. This in silico method is opposed to the alternative approach, ligand-based (pharmacophore), in which only ligands that are biochemically similar to the ones known to bind to the target are screened [16]. In this work, docking method was performed to predict the anti-inflammatory effect of newly synthesized compounds. At present, three COX isoenzymes COX-1, COX-2, and COX-3 are known of which COX-3 is splice variant of COX-1, a constitutive enzyme, found in most mammalian cells and COX-2 is undetectable in most normal tissues. COX-2 is an inducible enzyme abundant in cells with activated macrophages and other cells at sites of inflammation catalyzes the formation of prostaglandins, the messenger molecules in the process of inflammation and thromboxane from arachidonic acid derived from the cellular phospholipid bilayer by phospholipase A 2. The suppression of inflammation may be by the inhibition of COX or prostaglandins. The nonsteroidal anti-inflammatory drugs become main COX inhibitors. The classical COX inhibitors which are not selective inhibit all types of COX, causing peptic ulceration and dyspepsia. It is believed that such lack of selectivity is caused by the "dual-insult" of NSAIDs-direct irritation of the gastric mucosa and inhibition of prostaglandin synthesis by COX-1. Selectivity for COX-2 is the main feature of newer NSAIDS like celecoxib, rofecoxib, and other members of this drug class [17]. COX-2 is one of the well-known targets for the anti–inflammatory therapy. Selective inhibition of this enzyme overcomes the side effects associated with the traditional NSAIDs [18]. The reported 3-D QSAR models are mainly focused to a particular class of compounds and such models may not be useful to predict structurally diverse compounds [19]. It was reported a novel lead, phenothiazine for the inhibition of COX-2 enzyme using combined 3-D database searching and combinatorial chemistry methodologies [20]. The availability of several crystal structures of complexes of COX-2 with the inhibitors provides the possibility to apply structure based design techniques for the development of specific and potent inhibitors. Therefore, we thought of exploiting the structure based approach to design novel COX-2 inhibitors by docking studies combined with visualization of active site–ligand interactions. Visualization of docked inhibitors in COX-2 enzyme reveals that the carboxylate group of NSAIDs is located in a favourable position to interact with the guanidinium group of Arginine120 and OH of Tyrosine355.6. The standard compound, indomethacin, was docked into the active site of COX-2 successfully. Indomethacin forms 3 hydrogen bonds. One hydrogen bond with Tyr355 with a distance of 1.84 Å and 2 hydrogen bonds with Arg120 and the distances were found to be 2.23 and 2.56 Å. These are the key amino acids acting as a gate for ligand entrance to the COX active side (Figure 1). Molecular docking studies were done to provide insights of molecular binding modes of the tested molecules inside the pocket of COX-II enzyme using Discovery Studio 2.5 to evaluate the free energies and mode of binding of the proposed molecules with the active site of COX-II enzyme. The choice of the most promising molecules depends on the correct binding mode and the binding free energy (ΔG).
Preparation of the target protein
The protein target prepared and modelled according to the format requirements of the docking algorithms used. Thus the crystal structure of COX-II was downloaded from the protein data bank (PDB) (code 4COX) using Discovery Studio 2.5 software. Water molecules were removed from downloaded protein. Crystallographic disorders and unfilled valence atoms were corrected using alternate conformations and valence monitor options. Protein was subjected to energy minimization by applying CHARMM force fields for charge, and MMFF94 force field for partial charge. Inflexibility of structure is obtained by creating fixed atom constraint. The binding site of the protein was defined and prepared for docking. Ligand (Indomethacin) and the designed compounds 2D structures were sketched using ChemBioDraw Ultra 14.0 and saved in MDL-SDfile format. Sdfile opened, 3D structures were protonated and energy minimized by applying CHARMM force fields for charge, and MMFF94 force field for partial charge, then prepared for docking by optimization of the parameters.
Materials and Methods
General
All Melting points were measured in capillary tube on a Gallen lamp melting point apparatus and are uncorrected. The IR spectra were recorded on Nikolet IR 200FT IR spectrophotometer at pharmaceutical analytical Unit, Facility of Pharmacy, Al-Azhar University using KBr discs (λmax in cm-1). 1HNMR spectra were performed on Gimini 300 MHz, and Mercury 400 MHz and 100 MHz for 13C NMR, spectrometer at Chemical Warfare laboratories, Chemical Warfare Department, Ministery of Defence, Micro analytical Center of Cairo University and Zagazig University using TMS as internal standard and DMSO-d6 CDCl3 as solvent; the chemical shifts are reported in ppm (δ) and coupling constant (J) values are given in Hertz (Hz). Signal multiplicities are represented by s (singlet), d (doublet), t (triplet), q (quadruplet), and m (multiplet). All of the new compounds were analyzed for C, H and N and agreed with the proposed structures within ± 0.4% of the theoretical values by the automated CHN analyzer at the Regional Center for Mycology and Biotechnology, Faculty of Science, Cairo, Egypt. Mass spectra were recorded on Schimadzu GC/ MS-QP5050A spectrometer at the Regional Center for Mycology and Biotechnology, Faculty of Science, Cairo, Egypt. The purity of the compounds was checked by thin layer chromatography (TLC) on Merck silica gel 60 F254 precoated sheets.
Chemistry
1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (1): A solution of o-phenylenediamine (10.8 g, 0.01 mol) in ethanol (75 ml) was treated with a p-hydroxybezaldehyde (10.0 g, 0.01 mol) and the reaction mixture was grinding inmorter and then refluxed at 120°C for 3 hrs while that the reaction was controlled with TLC Technique (DCM: MEO 90/10 or ethyl acetate : n-hexan1:4), after complete of reaction the refluxed mixture was pour on ice and it leaved to form yellowish white precipitated that washed several times with water and was dried and crystallized from ethanol 95%. Yield 80%, m.p 1690C. IR (KBr, ν, cm-1): 3787(OH), 3062 (CH aromatic), 2985(CH aliphatic. 1HNMR (400MHz, [D6] CDCl3): δ=5.60 (s, 2H, CH2), 7.26-8.03 (m, 12H, Ar-H), 9.04 (s, 2H, OH, D2O exchangable). 13CNMR (DMSO-d6) δ (ppm): 51(CH2-N), 119-159(Ar-CH). MS (m/z): 316 (54, M+). Anal Calc. For C20H16N2 O2: C, 75.93; H, 5.10; N, 8.86. Found. C, 75.45; H, 5.32; N, 8.47.
General procedure for synthesis of 1-(4-Alkoxybenzyl)-2-(4-alkoxyphenyl)-1H-benzo[d] imidazole (2)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (250mg, 0.01mol) (1) and alkyl halide derivatives namely methyl bromide, ethyl iodide, bromo propane, butyl bromide, benzyl chloride allyl bromide (0.1 mol) and potassium carbonate (0.1 mol) and potassium iodide (0.002 mol) was relaxed in DMF (50 ml) until the reaction was completed (TLC) then the reaction mixture was poured on cold water, extracted and washed with diethyl ether, dried then crystallized from proper solvent .
1-(4-methoxybenzyl)-2-(4-methoxyphenyl)-1H-benzo[d]imidazole (2a)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and bromomethane (0.250 ml, 0.1 mol) and potassium carbonate (5.6 gm, 0.1 mol) and potassium iodide (0.32 mg, 0.002 mol) was refluxed in DMF (50 ml) for 8 hrs at 1500C, after complet of reaction (TLC) the reaction mixture was poured on cold water, extracted and washed with diethyl ether and crystallized from ethanol 95% . Yield 77%, m.p. 140-142°C. ). IR (KBr, ν, cm-1): 3050 (CH aromatic), 2985(CH aliphatic).1HNMR (400MHz, [D6] CDCl3): δ=3.80 (s, 6H, 2CH3), 5.65 (s, 2H, CH2), 7.26-8.03 (m, 12H, Ar-H). 13CNMR (DMSO-d6) δ (ppm):51(CH2-N), 55.9(2CH3), 115–160(Ar–CH). MS (m/z): 344 (18.6, M+). Anal. Calc. For C22H20N2O2: C, 76.72; H, 5.85; N,8.13. Found. C, 77.22; H, 5.61; N, 8.68.
1-(4-ethoxybenzyl)-2-(4-ethoxyphenyl)-1H-benzo[d]imidazole (2b)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and ethyl iodide (3.12 ml, 0.02 mol) and potassium carbonate (1.36 gm, 0.01 mol) and potassium iodide (0.64 mg, 0.002 mol) was refluxed in DMF (50 ml) for 8 hrs. at 900C, when the was completed (TLC) the reaction mixture was poured on ice cold water and extracted then washed with diethyl ether and crystallaized from ethanol 95%. Yield 95%, m.p. 150- 152°C. IR (KBr, ν, cm-1): 3050 (CH aromatic), 2985 (CH aliphatic), 1247 (CH2), 1410 (CH3). 1HNMR (400MHz, [D6] CDCl3): δ=1.40 (t, 6H, 2CH3, CH2CH3), 3.80 (q, 4H, 2CH2, CH2CH3), 5.63 (s, 2H, CH2), 7.26-8.03 (m,12H, Ar-H). 13CNMR (DMSO-d6) δ (ppm):12(2CH3), 65.2(2CH2), 54(CH2-N), 114–156(Ar-CH). MS (m/z): 372 (18.3, M+). Anal. Calc. For C24H24N2O2: C, 77.39; H, 6.49; N, 7.52. Found. C, 77.85; H, 7.01; N, 8.01.
1-(4-propoxybenzyl)-2-(4-propoxyphenyl)-1H-benzo[d]imidazole (2c)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and bromo propane (2.46 ml, 0.02 mol) and potassium carbonate (2.72 gm, 0.2mol) and potassium iodide (0.64 mg, 0.002 mol) was refluxed in DMF (50 ml) for 8 hrs. at 900C, finishing of the reaction (TLC) and the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%. Yield 85%, m.p. 118-120°C. IR (KBr, ν, cm-1): 3010 (CH aromatic), 2985 (CH aliphatic), 1516 (CH2), 1410 (CH3), 1247 (CH2). 1HNMR (400 MHz, [D6] CDCl3): δ=1.07 (t, 6H, 2CH3, CH2CH2CH3), 1.20 (m, 4H, 2CH2, CH2CH2CH3), 3.80 (t, 4H, 2CH2, CH2CH2CH3), 5.60(s, 2H, CH2), 7.26-8.03 (m, 12H, Ar-H). MS (m/z): 400 (32.2, M+). Anal. Calc. For C26H28N2O2: C, 77.97; H, 7.14; N, 6.99. Found. C, 78.05; H, 7.14; N, 7.08.
1-(4-butoxybenzyl)-2-(4-butoxyphenyl)-1H-benzo[d]imidazole (2d)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and butyl bromide (0.70 ml, 0.02 mol) and potassium carbonate (5.60 gm, 0.1mol) and potassium iodide (0.64 mg, 0.002 mol) was refluxed in DMF (50 ml) for 8 hrs. at 900C, finishing of the reaction (TLC) and the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%. Yield 87%, m.p. 98-99°C. IR (KBr, ν, cm-1): 3050 (CH aromtic), 2873 (CH aliphatic), 1516 (CH2), 1410 (CH3), 1247 (CH2). 1HNMR (400MHz, [D6] DMSO): δ (400 MHz, [D6] CDCl3): δ=0.95 (t, 6H, 2CH3, CH2CH2CH2CH3), 1.20 (m, 4H, 2CH2, CH2CH2CH2CH3), 2.20 (t, 4H, 2CH2, CH2CH2CH2CH3), 3.80 (t, 4H, 2CH2, CH2CH2CH2CH3), 5.60(s, 2H, CH2), 7.26-8.03 (m,12H, Ar- H). MS (m/z): 428 (17.5, M+). Anal. Calc. For C28H32N2O2: C, 78.47; H, 7.53; N, 6.54. Found. C, 78.04; H, 7.40; N, 6.12.
1-(4-benzoyloxybenzyl)-2-(4-benzoyloxyphenyl)-1H-benzo[d]imidazole (2e)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and benzoyl chloride (0.40 ml, 0.02 mol) and potassium carbonate (5.60 gm, 0.1mol) and potassium iodide (0.32 mg, 0.001 mol) was refluxed in DMF (50 ml) for 8 hrs. at 900C, after finishing of the reaction (TLC) the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%. Yield 75%, m.p. 150-152°C. IR (KBr, ν, cm-1): 3050 (CH aromatic), 1745 (C=O). 1HNMR (300 MHz, [D6] CDCl3): δ=5.60 (s, 2H, CH2), 7.22-8.21 (m,22H, Ar-H). MS (m/z): 524 (7.3, M+). Anal. Calc. For C34H24N2O4: C, 77.85; H, 4.61; N, 5.34. Found. C, 77.68; H, 5.10; N, 5.29.
1-(4-Allyloxybenzyl)-2-(4-methoxyphenyl)-1H-benzo[d]imidazole (2f)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and allyl bromide (2.42 ml, 0.05 mol) and potassium carbonate (2.72 gm, 0.1 mol) and potassium iodide (0.64 mg, 0.02 mol) was refluxed in DMF (50 ml) at 900C for 8 hrs, after finishing of the reaction (TLC) the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%. Yield 75%, m.p. 108-110°C. IR (KBr, ν, cm-1): 3050 (CH aromtic.), 2884 (CH aliphtic 1612 (C=C). 1HNMR (400 MHz, [D6] CDCl3): δ=5.17-5.28(m, 4H, CH2, allyl), 5.38(d, 4H, CH2, allyl, J=6 Hz), 5.60 (s, 2H, CH2), 6.15-6.09(m, 2H, CH, allyl), 7.26-8.03(m, 12H, Ar-H). 13CNMR (DMSO-d6) δ (ppm): 51.2(CH2-N), 71(CH2-CH), 114–157(Ar-CH). MS (m/z): 396 (14.2, M+). Anal. Calc. For C26H24N2O2: C, 78.76; H, 6.10; N, 7.07. Found. C, 78.68; H, 6.48; N, 6.99.
Methyl 2-(4-(1-(4-(1-methoxy-1-oxopropan-2-yloxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy) propanoate (3)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and methyl 2-chloropropanoate (2.44 ml, 0.02 mol) and potassium carbonate (2.72 gm, 0.02 mol) and potassium iodide (0.64 mg, 0.02 mol) was refluxed in DMF (50 ml) at 900C for 8 hrs, after finishing of the reaction (TLC) the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%. Yield 95%, m.p. 117-119°C. IR (KBr, ν, cm-1): 3041 (CH aromatic), 2958, 2880 (CH aliphatic), 1754 (C=O). 1HNMR (400 MHz, [D6] CDCl3): δ=1.50 (s, 6H, 2CH3CH), 3.50 (s, 6H, 2CH3-OCO), 4.80 (s, 2H, CH-O), 5.60 (s, 2H, CH2-N), 7.26-8.03(m,12H, Ar-H). 13CNMR (DMSO-d6) δ (ppm): 18(2CH3), 74(2CH), 51(2OCH3), 51(CH2-N), 112-154(Ar- CH), 171(2C=O). MS (m/z): 488.21 (9.82 %, M+), 77 (100%). Anal. Calc. For C28H28N2O6: C, 68.84; H, 5.78; N, 5.73. Found. C, 68.35; H, 5.98; N, 6.19.
Methyl 2-(4-(1-(4-(2-methoxy-2-oxoethoxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy)acetate (4a)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01 mol) (1) and methyl chloroacetate (2.16 ml, 0.02 mol) and potassium carbonate (2.72 gm, 0.02 mol) and potassium iodide (0.64 mg, 0.02 mol) was refluxed in DMF (50 ml) at 900C for 8 hrs, after finishing of the reaction (TLC) the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%. Yield 95%, m.p. 112- 114°C. IR (KBr, ν, cm-1): 3050 (CH aromatic), 2987 (CH aliphatic), 1641(C=O). 1HNMR (400 MHz, [D6] CDCl3): δ=3.05 (s, 6H, 2CH3, CH3-O), 4.80 (s, 4H, 2CH2, O-CH2), 5.60 (s, 2H, N-CH2), 7.26-8.03(m, 12H, Ar-H). 13CNMR (DMSO-d6) δ (ppm):49(2OCH3), 51(CH2-N), 65(2CH2C=O), 114-158(Ar-CH), 170(2C=O). MS (m/z): 460 (22.3, M+). Anal. Calc. For C26H24N2O6: C, 67.82; H, 5.25; N, 6.08. Found. C, 67.68; H, 5.01; N, 6.29.
Ethyl 2-(4-(1-(4-(2-ethoxy-2-oxoethoxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy)acetate (4b)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01 mol) (1) and ethyl 2-chloroacetate (2.44 ml, 0.02 mol) and potassium carbonate (2.72 gm, 0.02 mol) and potassium iodide (0.64 mg, 0.02 mol) was refluxed in DMF (50 ml) at 900C for 8 hrs, finishing of the reaction was confirmed with (TLC) then the reaction mixture was poured on cold water, filtered, washed with diethyl ether and crystallized from ethanol 95%. Yield 85%, m.p. 112-115°C. IR (KBr, ν, cm-1): 3041 (CH aromatic), 2958, 2880 (CH aliphatic), 1748 (C=O). 1HNMR (400MHz, [D6] CDCl3): δ=1.40 (t, 6H, 2CH3, CH2CH3), 3.50 (q, 4H, 2CH2, CH3CH2), 4.50 (s, 2H, CH2-O), 5.60 (s, 2H, CH2-N), 7.26-8.03(m, 12H, Ar-H). MS (m/z): 488 (11.7, M+). Anal. Calc. For C28H28N2O6: C, 68.84; H, 5.78; N, 5.73. Found. C, 68.44; H, 5.21; N, 6.19.
Isopropyl 2-(4-(1-(4-(2-isopropoxy-2-oxoethoxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy) acetate (4c)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01mol) (1) and isopropyl 2-chloroacetate (2.72 ml, 0.02 mol) and potassium carbonate (2.72 gm, 0.02 mol) and potassium iodide (0.64 mg, 0.02 mol) was refluxed in DMF (50 ml) at 90°C for 9 hrs, after complete of the reaction (TLC) the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%.Yield 65%, m.p. 100- 102°C. IR (KBr, ν, cm-1): 3048 (CH aromatic), 2811 (CH aliphatic), 1754 (C=O). 1HNMR (400 MHz, [D6] CDCl3): δ=1.32 (d, 12H, 4CH3, (CH3)2CH), 4.30 (m, 2H, (CH3)2CH), 4.80 (s, 4H, 2CH2, CH2O), 5.60 (s, 2H, CH2-N), 7.26-8.03 (m,12H, Ar-H). MS (m/z): 516 (2.5, M+). Anal. Calc. For C30H32N2O6: C, 69.77; H, 6.24; N, 5.42. Found. C, 70.17; H, 5.66; N, 5.89.
Isobutyl 2-(4-(1-(4-(2-isobutoxy-2-oxoethoxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy)acetate (4d)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01 mol) (1) and isobutyl 2-chloroacetate (3 ml, 0.02 mol) and potassium carbonate (2.72 gm, 0.02 mol) and potassium iodide (0.64 mg, 0.02 mol) was refluxed in DMF (50 ml) at 900C for 8 hrs, the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%.Yield 65%, m.p. 88-900C. IR (KBr, ν, cm-1): 3051 (CH aromatic), 2885 (CH aliphatic), 1754 (C=O). 1HNMR (400 MHz, [D6] CDCl3): δ=1.10 (d, 12H, 4CH3, (CH3)2CH), 1.54 (m, 2H, CH3CH), 2.4 (d, 4H, O-CH2CH), 2.9 (s, 4H, CH2-O), 5.60 (s, 2H, CH2-N), 7.26-8.03 (m,12H, Ar-H). MS (m/z): 544 (17.1, M+). Anal. Calc. For C32H36N2O6: C, 70.57; H, 6.66; N, 5.14. Found. C, 70.09; H, 6.97; N, 4.99.
Ethyl 4-(4-(1-(4-(4-ethoxy-4-oxobutoxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy)butanoate (4e)
A mixture of 1-(4-hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole (3.16 gm, 0.01 mol) (1) and ethyl 4-bromobutrate (4.18 ml, 0.02 mol) and potassium carbonate (2.72 gm, 0.02 mol) and potassium iodide (0.64 mg, 0.02 mol) was refluxed in DMF (50 ml) at 900C for 8 hrs, after finishing of the reaction (TLC) the reaction mixture was poured on cold water then filtered, washed with diethyl ether and crystallized from ethanol 95%.Yield 70%, m.p. 95-98°C. IR (KBr, ν, cm-1): 3008 (CH aromatic), 2885 (CH aliphatic), 1754 (C=O). 1HNMR (400MHz, [D6] CDCl3): δ=1.19 (t, 6H, 2CH3, CH3CH2), 2.20 (m, 4H, 2CH2, CH2CH2CH2), 2.30 (t, 4H, 2CH2, CH2CH2CO), 4.06 (t, 4H, 2CH2, O-CH2CH2), 4.18 (q, 4H, 2CH2, CH2CH3), 5.60 (s, 2H, CH2-N), 7.26-8.03 (m,12H, Ar-H). MS (m/z): 544 (12.2, M+). Anal. Calc. For C32H36N2O6: C, 70.57; H, 6.66; N, 5.14. Found. C, 70.80; H, 6.21; N, 5.17.
Potassium 4-(1-(4-oxidobenzyl)-1H-benzo[d]imidazol-2-yl)phenolate (5).
Depending on different of physical characters compound 5 was formed by refluxing of 1-(4-hydroxybenzyl)-2-(4- hydroxyphenyl)-1H-benzo[d]imidazole (10 gm, 0.01 mol) (1) and potassium hydroxide (1.12 g, 0.02 mol) in absolute ethanol (50 ml) for 2 hr upon cooling to room temperature a buff white precipitate product was obtained which was collected and crystallized from ethanol 95%. Yield 90%, m.p.>300°C.
2-(4-(1-(4-(1-hydrazinyl-1-oxopropan-2-yloxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy) propanehydrazide (6)
A mixture of 3 (46gm, 0.01) and hydrazine hydrate in ethanol (20 ml) was stirred well then heated at 500C for 6 hrs. The reaction mixture was cooled and the crude product was collected by filtration and washed with water and crystallized from ethanol 95%. Yield 70%, m.p. 95-98°C. IR (KBr, ν, cm-1): 3423 (NH2), 3210( NH), 3030 (CH aromatic), 2880 (CH aliphatic), 1750, 1740 (C=O). 1HNMR (400 MHz, [D6] CDCl3): δ=1.52(d, 6H, 2CH3), 1.90 (s, 4H, 2NH2, D2O exchangeable), 4.50(m, 2H, 2CH-O), 5.80 (s, 2H, CH2-N), 7.26-8.03 (m, 12H, Ar-H), 10,10 (s, 2H, 2NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm):18.2 (2CH3), 51(CH2-N), 113-157 (Ar-CH), 146 (HC=N), 174 (2C=O). MS (m/z): 488 (6.7, M+). Anal. Calc. For C26H28N6O4: C, 63.92; H, 5.78; N, 17.20. Found. C, 63.84; H, 6.13; N, 17.47.
General procedure for synthesis of substituted benzylidene -2-(4-(1-(4-(1-(-2-(substituted benzylidene) hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy) propanehydrazide 7a-g
Equimolar amounts of hydrazide 6 (4.88 g, 0.002 mol) and appropriate aldehydes namely ( benzaldehyde, o-chlorobenzaldehyde, p-chlorobenzaldehyde, o-hydroxybenzaldehyde, p-hydroxybenzaldehyde, o-methoxybenzaldehyde, p-methoxybenzaldehyde) (0.002 mol) were refluxed in ethanol 95% and acetic acid (1 ml) for apropriate time and the reaction was followed up by TLC, the mixture was cooled, filtered and crystallized from ethanol %.
N'-benzylidene-2-(4-(1-(4-(1-(-2-benzylidenehydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1H-benzo[d]imidazol-2-yl) phenoxy)propanehydrazide (7a)
Yield 71%, m.p. 250-252°C. IR (KBr, ν, cm-1): 3322(NH), 3048 (CH aromatic), 2964 (CH aliphatic), 1659 ( C=O), 1609 (HC=N azomethine). 1HNMR (400MHz, [D6] DMSO): δ=1.20 (s, 6H, 2CH3), 4.70 (q, 2H, 2CH-O), 5.60 (s, 2H, CH2-N), 7.26-8.15 (m, 22H, Ar-H), 9.70 (s, 1H, N=CH), 9.93 (s, 1H, N=CH), 10,17 (s, 2H, 2NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm):18(2CH3), 77(2CH), 51(CH2-N), 114-157 (Ar-CH), 148 (HC=N), 175 (2C=O). MS (m/z): 664 (2.8, M+). Anal. Calc. For C40H36N6O4: C, 72.27; H, 5.46; N, 12.64. Found. C, 62.24; H, 5.43; N, 12.52.
N'-2-chlorobenzylidene-2-(4-(1-(4-(1-(-2-(2-chlorobenzylidene)hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1Hbenzo[ d]imidazol-2-yl)phenoxy)propanehydrazide (7b)
Yield 72%, m.p. 247-249°C. IR (KBr, ν, cm-1): 3417(NH), 3089 (CH aromatic), 2980 (CH aliphatic), 1659 (C=O), 1609 (HC=N azomethine). 1HNMR (400MHz, [D6] DMSO): δ=1.30 (s, 6H, 2CH3), 4.65(q, 2H, 2CH-O), 5.62 (s, 2H, CH2-N), 7.24-8.16 (m, 20H, Ar-H), 9.72 (s, 1H, N=CH), 9.80(s, 1H, N=CH), 10,12 (s, 2H, 2NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm):17.7 (2CH3), 51 (CH2-N), 75(2CH), 112-158 (Ar-CH), 147 (HC=N), 177(2C=O). MS (m/z): 732 (4.11, M+), 734 (12.20, M+2), Anal. Calc. For C40H34N6O4: C, 65.49; H, 4.67; N, 11.46. Found. C, 65.93; H, 4.89; N, 11.59.
N'-4-chlorobenzylidene-2-(4-(1-(4-(1-(-2-(4-chlorobenzylidene)hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1Hbenzo[d]imidazol-2-yl)phenoxy)propanehydrazide (7c)
Yield 80%, m.p. 258-260°C. IR (KBr, ν, cm-1): 3281(NH), 3089 (CH aromatic), 2980 (CH aliphatic), 1662 (C=O), 1612 (HC=N azomethine). 1HNMR (400MHz, [D6] DMSO): δ=1.23 (s, 6H, 2CH3), 4.57 (q, 2H, 2CH-O), 5.46 (s, 2H, CH2-N), 7.18-8.03 (m, 20H, Ar-H), 9.70 (s, 1H, N=CH), 9.86 (s, 1H, N=CH), 10,04 (s, 2H, 2NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 18.5(2CH3), 51(CH2-N), 77(2CH), 112-158 (Ar-CH), 148 (HC=N), 176 (2C=O). MS (m/z): 732 (1.12, M+), 734 (3.35, M+2). Anal. Calc. For C40H34N6O4: C, 65.49; H, 4.67; N, 11.46. Found. C, 65.13; H, 5.02; N, 11.71.
N'-2-hydroxybenzylidene-2-(4-(1-(4-(1-(-2-(2-hydroxybenzylidene)hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1Hbenzo[ d]imidazol-2-yl)phenoxy)propanehydrazide (7d)
Yield 71%, m.p. 272-274°C. ). IR (KBr, ν, cm-1): 3357(NH), 3040 (CH aromatic), 2980 (CH aliphatic), 1665 (C=O), 1602 (HC=N azomethine). 1HNMR (400MHz, [D6] DMSO): δ=1.20 (s, 6H, 2CH3), 4.70(q, 2H, 2CH-O), 5.60 (s, 2H, CH2-N), 7.26-8.15 (m,20H, Ar-H), 9.30 (s, 1H, N=CH), 9.43 (s, 1H, N=CH), 9,97(s, 2H, 2NH, D2O exchangeable), 12.1 (s, 1H, o-OH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm):18.9(2CH3), 51(CH2-N), 77.2 (2CH), 111-157 (Ar-CH), 147 (HC=N), 176(2C=O), MS (m/z): 696 (5.1, M+). Anal. Calc. For C40H36N6O6: C, 68.95; H, 5.21; N, 12.06. Found. C, 68.81; H, 5.21; N, 12.55.
N'-4-hydroxybenzylidene-2-(4-(1-(4-(1-(-2-(4-hydroxybenzylidene)hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1Hbenzo[ d]imidazol-2-yl)phenoxy)propanehydrazide (7e)
Yield 65%, m.p. 270-272°C. IR (KBr, ν, cm-1): 3340(NH), 3030 (CH aromatic), 2920 (CH aliphatic), 1654 (C=O), 1608 (HC=N azomethine). 1HNMR (400MHz, [D6] DMSO): δ=1.22(s, 6H, 2CH3), 4.68(q, 2H, 2CH-O), 5.54 (s, 2H, CH2-N), 7.26-8.30(m, 20H, Ar-H), 9.59(s, 1H, N=CH), 9.64(s, 1H, N=CH), 10,07(s, 2H, 2NH, D2O exchangeable), 12.1 (s, 1H, p-OH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm):19.2 (2CH3), 51(CH2-N), 76.4 (2CH), 114-156 (Ar–CH), 146 (HC=N), 174(2C=O). MS (m/z): 696 (3.9, M+). Anal. Calc. For C40H36N6O6: C, 68.95; H, 5.21; N, 12.06. Found. C, 68.93; H, 5.22; N, 12.33.
N'-2-methoxybenzylidene-2-(4-(1-(4-(1-(-2-(2-methoxybenzylidene)hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1Hbenzo[ d]imidazol-2-yl)phenoxy)propanehydrazide (7f)
Yield 60%, m.p. 262-264°C. IR (KBr, ν, cm-1): 3322 (NH), 3048 (CH aromatic), 2964 (CH aliphatic), 1659 (C=O), 1609 (HC=N azomethine). 1HNMR (400 MHz, [D6] DMSO): δ=1.17 (s, 6H, 2CH3), 3.45 (s, 3H, OCH3), 4.40 (q, 2H, 2CH-O), 5.55 (s, 2H, CH2-N), 7.26-8.24 (m, 20H, Ar-H), 9.50 (s, 1H, N=CH), 9.59 (s, 1H, N=CH), 10,05 (s, 2H, 2NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 18 (2CH3), 51 (CH2-N), 54 (2OCH3), 75 (2CH), 112-157 (Ar-CH), 147.4 (HC=N), 173 (2C=O). MS (m/z): 724 (9.3, M+). Anal. Calc. For C42H40N6O6: C, 69.60; H, 5.56; N, 11.59. Found. C, 70.01; H, 5.42; N, 11.20.
N'-4-methoxybenzylidene-2-(4-(1-(4-(1-(-2-(4-methoxybenzylidene)hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1Hbenzo[ d]imidazol-2-yl)phenoxy)propanehydrazide (7g)
Yield 60%, m.p. 260-262°C. IR (KBr, ν, cm-1): 3322(NH), 3048 (CH aromatic), 2964 (CH aliphatic), 1659 (C=O), 1609(HC=N azomethine). 1HNMR (400 MHz, [D6] DMSO): δ=1.10 (s, 6H, 2CH3), 3.49(s, 3H, OCH3), 4.32 (q, 2H, 2CH-O), 5.51 (s, 2H, CH2-N), 7.26-8.31(m, 20H, Ar-H), 9.50 (s, 1H, N=CH), 9.57 (s, 1H, N=CH), 10,11(s, 2H, 2NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 19.2 (2CH3), 51 (CH2-N), 55.8 (2OCH3), 77 (2CH), 111-154 (Ar- CH), 146.4 (HC=N), 176 (2C=O). MS (m/z): 724 (6.2, M+). Anal. Calc. For C42H40N6O6: C, 69.60; H, 5.56; N, 11.59. Found. C, 69.61; H, 6.01; N, 11.92.
General procedure for synthesis of compounds 8a-c
A mixture of hydrazide 6 (4.88 g, 0.002 mol) and appropriate amount of anhydride namely (succinic anhydride, maleic anhydride and phathalic anhydride) (0.002 mol) were refluxed in acetic acid for appropriate time and the reaction was followed up by TLC, the mixture was cooled, filtered and crystallized from ethanol 95%.
2-(4-(1-(4-(1-oxo-1-(pyrrolidin-1-ylamino)propan-2-yloxy)benzyl)-1H-benzo[d]imidazol-2-yl)phenoxy)-N- (pyrrolidin-1-yl)propanamide (8a)
Yield 70%, m.p. 212-214°C. IR (KBr, ν, cm-1): 3240 (NH), 3048 (CH aromatic), 2910 (CH aliphatic), 1645 (C=O). 1HNMR (400MHz, [D6] DMSO): δ=1.50 (s, 6H, CH3), 1.89 (p, 8H, CH2CH2-pyrolidine), 3.17 (t, 8H, CH2Npyrolidine), 4.66 (q, H, CH-O), 5.72 (s, 2H, CH2-N), 6.86-7.98 (m, 12H, Ar-H), 10,90(s, 2H, NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 18.2(2CH3), 23 (2CH2-pyrolidine), 51 (2CH2-N), 57.3 (2CH2-N-pyrolidine), 77 (2CHO), 113-156 (Ar-CH), 168.1(2C=O). MS (m/z): 596 (0.7, M+). Anal. Calc. For C34H40N6O4: C, 68.43; H, 6.76; N, 14.08. Found. C, 68.02; H, 6.88; N, 14.13.
N-(2,5-dihydro-1H-pyrrol-1-yl)-2-(4-(1-(4-(1-(2,5-dihydro-1H-pyrrol-1-ylamino)-1-oxopropan-2-yloxy)benzyl)- 1H-benzo[d]imidazol-2-yl)phenoxy)propanamide (8b)
Yield 71%, m.p. 230-232°C. IR (KBr, ν, cm-1): 3277(NH), 3051 (CH aromatic), 2830 (CH aliphatic), 1647 (C=O). 1HNMR (400 MHz, [D6] DMSO): δ=1.47 (s, 6H, CH3), 3.25 (d, 8H, CH2N-dihyropyrolidine), 4.75 (q, 2H, CHO), 5.80 (s, 2H, CH2-N), 6.01 (q, 4H, CH-dihydropyrolidine), 6.86-8.02 (m, 12H, Ar-H), 10,93 (s, 2H, NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 18.9 (2CH3), 52 (2CH2-N-dihydropyrolidine), 77 (2CH-O), 114–158 (Ar-CH), 170 (2C=O). MS (m/z): 592 (1.3, M+). Anal. Calc. For C34H36N6O4: C, 68.90; H, 6.12; N, 14.18. Found. C, 68.69; H, 6.85; N, 14.31.
N-(isoindolin-2-yl)-2-(4-(1-(4-(1-(isoindolin-2-ylamino)-1-oxopropan-2-yloxy)benzyl)-1H-benzo[d]imidazol-2-yl) phenoxy)propanamide (8c)
Yield 68%, m.p. 220-222°C. IR (KBr, ν, cm-1): 3293(NH), 3055 (CH aromatic), 2915(CH aliphatic), 1653 (C=O). 1HNMR (400MHz, [D6] DMSO): δ=1.47(s, 6H, CH3), 3.74(s, 8H, CH2N-dihydrropyrolidine), 4.70(q, 2H, CH-O), 5.82 (s, 2H, CH2-N), 6.87-8.23(m, 20H, Ar-H), 11,05 (s, 2H, NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 19(2CH3), 51(2CH2-N), 66(2CH2-N-dihydropyrolidine), 77(2CH-O), 114-155(Ar-CH), 170(2C=O). MS (m/z): 692 (3.8, M+). Anal. Calc. For C42H40N6O4: C, 72.81; H, 5.82; N, 12.13. Found. C, 72.89; H, 5.88; N, 12.22.
2-(2-(4-(1-(4-(1-oxo-1-(2-(phenylcarbamoyl)hydrazinyl)propan-2-yloxy)benzyl)-1H-benzo[d]imi-dazol-2-yl) phenoxy)propanoyl)-N-phenylhydrazinecarboxamide (9)
Yield 70%, m.p. 158-160°C. IR (KBr, ν, cm-1): 3293(NH), 3310, 3219, 3159(NH), 3042 (CH aromatic), 2823(CH aliphatic), 1645 (C=O). 1HNMR (400 MHz, [D6] DMSO): δ=1.44 (s, 6H, CH3), 4.72(q, 2H, CH-O), 5.66 (s, 2H, CH2-N), 6.81-8.11 (m, 22H, Ar-H), 8.66, 7.81(s, 4H, NH, D2O exchangeable), 10.92 (s, 1H, NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 18.7 (2CH3), 51 (2CH2-N-), 77 (2CH-O), 112-168 (Ar-CH), 171 (2C=O). MS (m/z): 726 (15.7, M+). Anal. Calc. For C40H38N8O6: C, 66.10; H, 5.27; N, 15.42. Found. C, 66.41; H, 4.92; N, 15.79.
N-ethyl-2-(2-(4-(1-(4-(1-(2-(ethylcarbamothioyl)hydrazinyl)-1-oxopropan-2-yloxy)benzyl)-1H-benzo[d]imidazol- 2-yl)phenoxy)propanoyl)hydrazinecarbothioamide (10)
Yield 70%, m.p. 158-160°C. IR (KBr, ν, cm-1): 3293 (NH), 3300, 3211, 3112(NH), 3042 (CH aromatic), 2823 (CH aliphatic), 1645 (C=O). 1HNMR (400 MHz, [D6] DMSO): δ=1.03 (t, 6H, CH3CH2), 1.48 (s, 6H, CH3CH), 3.16 (q, 4H, CH3CH2), 4.68(q, 2H, CH-O), 5.70 (s, 2H, CH2-N), 6.80-8.03(m, 12H, Ar-H), 8.30, 7.79 (s, 4H, NH, D2O exchangeable), 11.02(s, 1H, NH, D2O exchangeable). 13CNMR (DMSO-d6) δ (ppm): 15.3 (2CH3), 18.7 (2CH3), 32.8 (2CH2), 52 (2CH2-N-), 76 (2CH-O), 112–166.7(Ar–CH), 170.3(2C=O). MS (m/z): 726 (15.7, M+). Anal. Calc. For C32H38N8O4S2: C, 57.99; H, 5.78; N, 16.91. Found. C, 58.28; H, 5.91; N, 16.56.
Results and discussion of molecular modelling
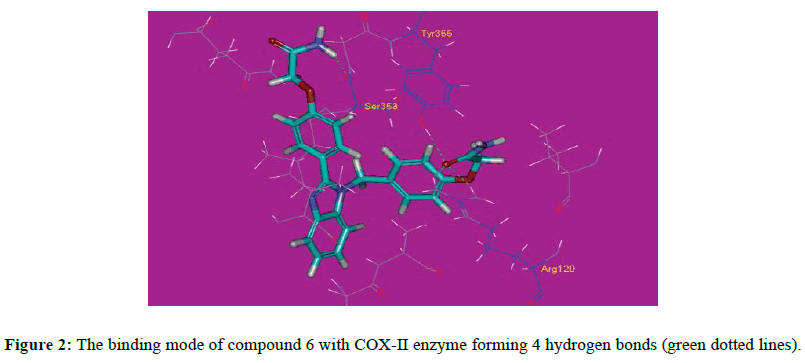
The obtained results indicated that all studied ligands have a similar position and orientation inside the binding site of COX-II enzyme. All the designed molecules showed good binding energies ranging from -27.70 to -50.37 kcal/mol (Table 1). The proposed binding mode of the ligand, indomethacin, has binding free energy of -49.21 kcal/mol and its important interaction with the residues at the active site of COX-II enzyme is shown in (Figure 1). The proposed binding mode of compound 6 (affinity value of -49.45 kcal/mol and 4 H-bonds) is virtually the same as that of indomethacin with an extra binding site, where the carbonyl of one side chain formed two hydrogen bonds with distances of 1.87 and 1.85 A˚ with the polar residue Arg120. Additionally, the same carbonyl formed a hydrogen bond with the hydroxyl group of Tyr355 at 1.92 A˚. The NH2 of the other side chain formed a hydrogen bond with the carbonyl group of Ser353 with a distance of 2.15 A˚ (Figure 2). It also forms Pi-Sigma interaction with Ser353. These bonds and the correct binding mode explain the high energy of binding and the good biological activity of compound 6.
| Comp. No. | Binding free energy (kcal/mol) | Comp. No | Binding free energy (kcal/mol) |
|---|---|---|---|
| Indomethazine | -49.21 | Indomethazine | -49.21 |
| 2a | -42.64 | 6 | -39.8 |
| 2b | -42.6 | 7a | -36.63 |
| 3 | -46.67 | 7b | -29.1 |
| 4c | -46.55 | 8a | -24.5 |
| 4e | -46.37 | 8b | -20.9 |
Table 1: The calculated ΔG (binding free energies) of the synthesized compounds
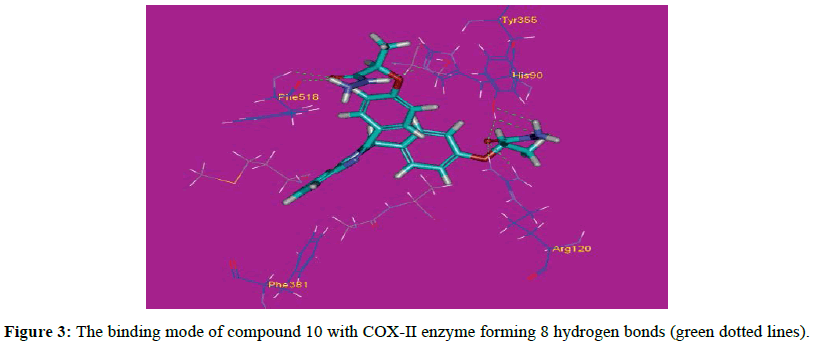
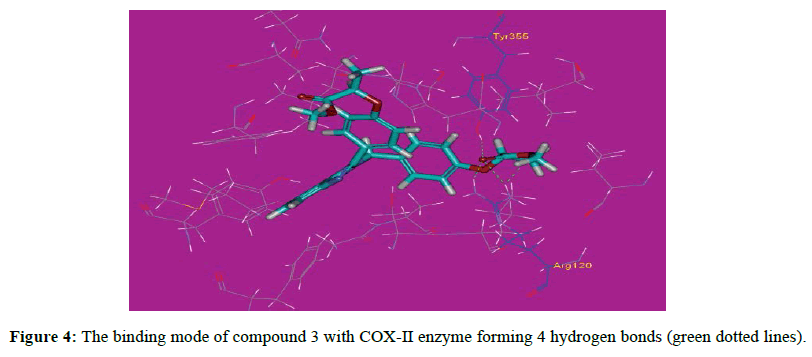
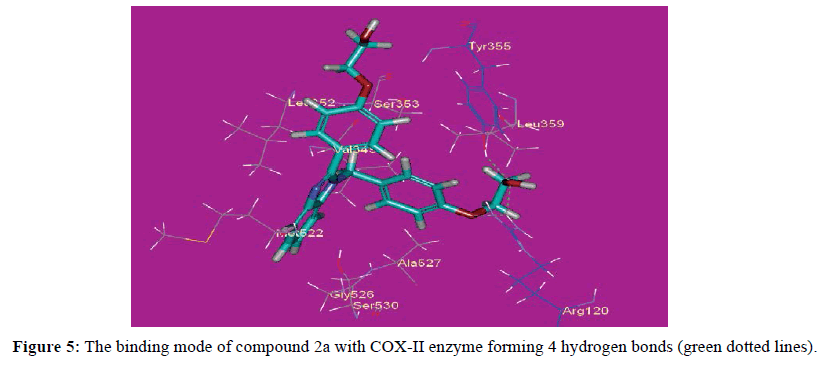
The proposed binding mode of compound 10 (affinity value of -50.37 kcal/mol and 8 H-bonds) is virtually the same as that of indomethacin with extra binding sites, where the carbonyl of one side chain formed two hydrogen bonds with distances of 2.20 and 2.12 A˚ with the polar residue Arg120. The hydroxyl proton of Tyr355 formed two hydrogen bonds with the NH2 group and the carbonyl of compound 10 at distances 2.44 and 2.11 A˚ respectively, while the hydroxyl oxygen of Tyr355 formed a hydrogen bond with the proton of the NH2 group at 2.37 A˚. Moreover, compound 10 a hydrogen bond with His90 and two hydrogen bonds with Phe518 with distances of 2.05, 1.93 and 2.44 A˚ respectively (Figure 3). These bonds and the correct binding mode explain the high energy of binding and the good biological activity of compound 10. The proposed binding mode of compound 3 (affinity value of -46.67 kcal/mol and 4 H-bonds) is virtually the same as that of indomethacin with extra binding sites, where the carbonyl of one side chain formed two hydrogen bonds with distances of 2.20 and 2.12 A˚ with the polar residue Arg120. Moreover, the ester oxygen formed a hydrogen bond with a distance of 2.15 A˚ with Arg120. The hydroxyl group of Tyr355 attached to compound 3 with a hydrogen bond at 2.76 A˚ (Figure 4). These bonds and the correct binding mode explain the high energy of binding and the good biological activity of compound 3. The proposed binding mode of compound 2a(affinity value of -42.64 kcal/mol and 3 H-bonds) is virtually the same as that of indomethacin, where the oxygen atoms of one side chain formed two hydrogen bonds with distances of 2.14 and 2.29 A˚ with the polar residue Arg120. Moreover, one oxygen atom formed a hydrogen bond with Tyr355 at 2.54 A˚ (Figure 5). It also forms Pi-Sigma interaction with Ser353.These bonds and the correct binding mode explains the high energy of binding and the good biological activity of compound 2a.
Results and Discussion
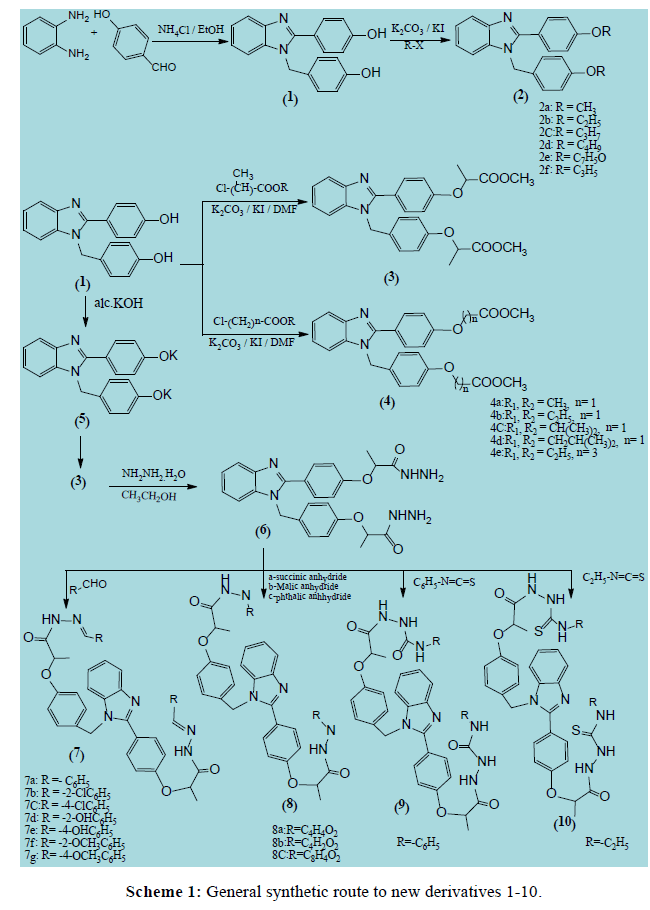
The synthetic method adopted to obtain the newly key starting benzimidazole 1 that was prepared via condensation of o-Phenelenediamine with p-hydroxybenzaldehyde (Scheme 1) and its structure were established on the basis of elemental analysis and spectral data where the IR spectra of these compounds showed characteristic absorption band at 3787 corresponding to OH functionality that also confirmed by the 1H NMR spectrum that exhibit significant singlet of two protons at 9.04 ppm (exchangeable with D2O) due to this two OH. Moreover, this condensation also confirmed by the presence of singlet signal at 5.6 ppm corresponding to aliphatic CH2-bridge. Salt formation of benzimidazols 2a-f was obtained upon treatment of 1 with different alkyl derivatives and its structures were confirmed on the basis of elemental analysis and spectral data where 1HNMR of compound 2a characterized by singlet of six proton corresponding to two methoxy group and allyl protons of compound 2f appear at δ 5.17-5.38 ppm. In conclusion, a facile general method for synthesis of compound 3 or 4a-e depending on the reaction of 1 with a suitable alkyl ester to afford of corresponding benzimidazol ester 3 and 4a-e. The IR spectra of these compounds showed disappearance (OH) absorption band confirming the formation of the desired benzimidazol esters. In addition, the appearance of (C=O) absorption band between 1748-1754 cm-1 confirm the obtained benzimdazols structure. Moreover, 13CNMR spectrum displayed significant signals around δ 170-171 ppm for (C=O). Hydrazinolysis of compound 3 with hydrazine hydrate afford compound 6, where its structure was confirmed in the 1HNMR spectrum by appearance of singlet signal of four protons at δ 1,9 ppm corresponding to two NH2( D2O exchangeable) and another singlet signal of two protons that confirm the presence of two NH ( D2O exchangeable) at δ 10.10 ppm. When compound 6 react with different substituted aldehydes it afford new benzimidazol 7a-g compounds and the structure of the resulting derivatives was confirmed from IR spectra by appearance of stretching band in the region 1654-1665 cm-1 belong the amidic C=O stretching that proved by 13C NMR at δ 173-177 in addition to, 1H NMR spectra of 7 showed the presence of one singlet at δ 9.30-9.93 ppm due to the presence of benzylidene CH and another singlet around δ 10 ppm due to two NH ( D2O exchangeable). Reaction of benimidazol 6 with the appropriate acidanhydride (succinic anhydride, maleic anhydride/or phthalic anhydride) afford compounds 8a-c and its IR spectra showed disappearance of stretching band at 3423 due toNH2 in addition, to mass spectra showed a peak at m/z 595 (0.7, M+), m/z 592 (1.3, M+), m/z 692 (3.8, M+) was observed for compounds 8a, 8b and 8c respectively. The studying of the reaction of compound 6 with phenyl isothiocyanate and ethyl isothiocyanate involved a nucleophilic attack to produce the acyclic derivatives 9 and 10 respectively. The structure of this compound was proven by the presence of three singlets at δ (7,81, 8.66, 10.92) and (7,79, 8.30, 11.02) ppm corresponding to three NH ( D2O exchangeable) and 13C NMR, Mass spectral data and elemental analysis results are in agreement with the proposed structures to compound 9 and compound 10.
Pharmacology
In vivo anti-inflammatory activity: Adult healthy female Wister albino rats of weighting between 160-200 gm were used for the study. The animals were house in standard condition (temperature 24 ± 2 with 50-60% relative humidity and a 12 hours light dark cycle). The entire animal had free access to water and normal diet. The study was approved by Institutional Animal Ethical Committee (IAEC) and was in accordance with the guideline of the Committee for the Purpose of Control and Supervision of Experimental Animal (CPCSEA). The initial paw volume of each rat was noted by using of Caliper. Eighty four animals were used in this study and divided into 14 groups (six animals per each). Group-1 was served for Carrageenan injection in the right hind paw.Group-2 was received for indomethacin at a dose of 12.5 mg/kg body weight, whereas group 3-14 received the test samples. After one hour from the compounds treatment orally at a dose of 100 mg/kg, 1% w/v from Carrageenan solution (0.1 ml/paw) was injected subcutaneously into the plantar surface of the right hind paw of the rat. The paw volume of the left legs that considered as control for each animal in Carrageenan, standard & tested groups were measured with the help of official caliper during the time intervals of 1, 3, 6, 12 and 24 h after Carrageenan administration. Percentage protection (or inhibition) was calculated by using the formula, % protection=(1-Vt/Vc) × 100, where: Vt is the mean increase in the paw volume in the test animals group, Vc is the mean increase in the paw volume in the control group (in anti-inflammatory study).
Anti-inflammatory of indomethacin, and tested compounds in Carrageenan injected rats
Data in Table 2 show that injection of carrageenan in right hind paw (S.C) in a dose of 1% w/v 0.1 ml caused a significant increase in right paw volume by about (261.5%, 400%, 315.4% 311.5% and 203.8% after 1, 3, 6, 12 and 24 h respectively when compared to volume of left leg, while pre-treatment with indomethacin (i.p.) in a dose of 12.5 mg/kg caused a significant reduction in right paw volume by (68%, 256.1%, 189.6% 192.2 % and 100.5%), when compared to Carrageenan injected group at 1, 3, 6, 12 and 24 h respectively, the compounds (2e and 10) shows a significant reduction in right hind paw volume by (120,6% and 128.5%), when compared to Carrageenan injected group at end of experiment (24 h) respectively taken into consideration that this two compounds had little activity than indomethacin. 3, 4c and 6 had an intermediate reduction in right paw volume by (151.7%, 150% and 160.7%), when compared to carrageenan injected group at 24 h respectively. The following compounds 4c, 2c, 7a, 7b, 8a and 8b had very low activity when compared to standard drug and also nearly the same volume of right hind paw by (183.3%, 200% 222.2%, 214.2, 215.3 and 204%), when compared to carrageenan injected group at 24 h respectively, while the last compound 2b had no activity at the end of experiment whereas during early interval had good activity.
| Drug | Dose | Left-leg | Right-leg 1 h | Right-leg 3 h | Right-leg 6 h | Right-leg 12 h | Right-leg 24 h |
|---|---|---|---|---|---|---|---|
| Carrageenan | 0.1 ml | 0.26 | 0.68 | 1.04 | 0.82 | 0.81 | 0.53 |
| Indomethacin | 12.5 mg | 0.31 | 0.6 | 0.44 | 0.39 | 0.37 | 0.32 |
| 6 | 100 mg | 0.28 | 0.6 | 0.68 | 0.53 | 0.49 | 0.45 |
| 10 | 100 mg | 0.28 | 0.5 | 0.7 | 0.47 | 0.41 | 0.36 |
| 4c | 100 mg | 0.26 | 0.4 | 0.75 | 0.64 | 0.52 | 0.39 |
| 4e | 100 mg | 0.29 | 0.54 | 0.63 | 0.55 | 0.5 | 0.44 |
| 3 | 100 mg | 0.3 | 0.74 | 0.87 | 0.74 | 0.65 | 0.55 |
| 2e | 100 mg | 0.29 | 0.62 | 0.81 | 0.9 | 0.62 | 0.35 |
| 2b | 100 mg | 0.27 | 0.44 | 0.76 | 0.68 | 0.58 | 0.47 |
| 2c | 100 mg | 0.26 | 0.48 | 0.76 | 0.69 | 0.6 | 0.52 |
| 7a | 100 mg | 0.27 | 0.8 | 0.94 | 0.96 | 0.78 | 0.6 |
| 7b | 100 mg | 0.28 | 0.66 | 0.98 | 0.97 | 0.78 | 0.6 |
| 8a | 100 mg | 0.26 | 0.77 | 0.89 | 0.79 | 0.67 | 0.56 |
| 8b | 100 mg | 0.25 | 0.7 | 0.8 | 0.77 | 0.64 | 0.51 |
Table 2: In vivo Anti-inflammatory activity
Evaluation of ulcer index
Non-steroidal anti-inflammatory drugs model was employed to evaluate the ulcer index of indomethacin and tested compounds. Experimental Animal Forty two adult healthy female Wister albino rats of weighting between 160- 200 gm were used for the study. The animal in all the groups were kept for 24 h fasting after that the animals were divided into seven groups (each contain 6 animals) as follow: Group-I (Control):Distilled water , Group-II (Standard): Indomethacin (25 mg/Kg) orally, Group-III: 2a (300 mg/kg) orally, Group-IV: 4c (300 mg/kg) orally, Group-V:2b (300 mg/kg) orally, Group-VI:6 (300 mg/kg) orally, Group-VII:2a (300 mg/kg) orally Scoring of ulcer 0=Normal colored stomach 0.5=Red colouration, 1=Spot ulcer, 1.5=Haemorrhagic streaks, 2=Ulcers ≥, 3 but ≤ 5, 3=Ulcers>5 .And Calculation of ulcer Index 15:,U1=UN + US + UP × 10-1
U1=Ulcer Index, UN=Average of number of ulcer per animal, US=Average of severity score, UP=Percentage of animal with ulcer.
Theoretical discussion
In the present study, the oral administrations of compounds in adult female albino rats were evaluated for administration of ulcer index. The tested compounds were used at the dose of 300 mgl/Kg and produced significant changes in gastric lesion as compared by indomethacin induced ulcer. Compound 8b produce gastric mucosal lesions similar to that induced by indomethacin or slightly higher, whereas oral administration of compounds 4c, 4b and 4a produce very minor gastric mucosal lesions when they compared to indomethacin. On the other hand compound 6 did not cause any gastric mucosal lesions in the rat stomach at the dose (300 mg/Kg) indicating that this compound is devoid of gastric irritant properties. Regarding in-vivo anti-inflammatory activity the present study showed that, all the newly synthesized compounds were evaluated for their in-vivo anti-inflammatory activity and compared to indomethacin as a reference standard and it was measured before 1, 3, 6, 12 and 24 h and after carrageenan injection. per cent of oedema inhibition was calculated as a regard to the percentage of the changes of indomethacin and tested compounds, it was observed that the compounds 8b, and 10 had shown good activity at all-time points, and the compounds 4c, 3 and 6 had shown moderate activity, while the compounds 4e, 2c, 7a, 7b, 2b and 2a shown least activity, Hence other derivatives 2b have no anti-inflammatory activity and failed to inhibit oedema that is run with safety on gastric mucosa for this compounds. On the other hand we notice that the following compounds 10, 4c, 3, 2b and 2c had the maximal effect that observed at 1 h time period after carrageenan.
Conclusion
A new series of benzimidazol 1-10 were synthesized and screened for their anti-inflammatory and analgesic activities. From the data obtained in the Tables 3 and 4 it found that Compound 6 exhibit potent anti-inflammatory and analgesic activities and superior GIT safety profile in experimental rats in comparison to indomethacin as the reference drug. Molecular docking studies further supported the activity of 6 and further helped in understanding the various interactions between ligands and enzyme active sites. Based on the findings of these preclinical results, further studies need to be carried out to investigate the other specifications, such as in vitro assays, chronic ulcerogenicity studies and toxicological studies.
| Group | Dose mg/kg | Ulcer index |
|---|---|---|
| Control | - | - |
| Indomethacin | 20 | 6.65 ± 0.41 |
| 8b | 300 | 6.84 ± 0.43 |
| 4c | 300 | 1.02 ± 0.26* |
| 2b | 300 | 1.15 ± 0.30* |
| 6 | 300 | 0.72 ± 0.15* |
| 2a | 300 | 1.92 ± 0.45* |
Table 3: Effect of indomethacin and tested compounds-induced ulcer in rats
| Drug | Dose | Left leg | Right leg h |
|---|---|---|---|
| Carrageenan | 0.1 ml | 0.93 | 1.27 |
| Indomethacin | 12.5 mg | 0.97 | 0.98 |
| 6 | 100 mg | 0.95 | 1.25 |
| 10 | 100 mg | 1 | 1.05 |
| 4c | 100 mg | 0.9 | 1.15 |
| 4e | 100 mg | 1.1 | 1.25 |
| 3 | 100 mg | 1.15 | 1.3 |
| 2e | 100 mg | 0.95 | 1.25 |
| 2b | 100 mg | 1 | 1.3 |
| 2c | 100 mg | 0.85 | 1.3 |
| 7a | 100 mg | 1.1 | 1.35 |
| 7b | 100 mg | 1 | 1.25 |
| 8a | 100 mg | 0.9 | 1.1 |
| 8b | 100 mg | 0.75 | 1 |
Table 4: Weight of legs
Acknowledgements
The authors would like to express their sincere thanks to Dr. Ahmad Mansour, Pharmacology Department, Faculty of Pharmacy Al-Azher University, Cairo, Egypt, for carrying out the in-vivo analgesic and anti-inflammatory activities.
References
- Wilson LM, Price SA, Wilson LM (2003) Pathophysiology: Clinical Concepts of Disease Processes, Mosby, St. Louis, Missouri, pp: 44-61.
- Dannhardt G, Kiefer W (2001) Cyclooxygenase inhibitors--current status and future prospects. Eur J Med Chem 36: 109-126.
- Bergh MS, Budsberg SC (2005) The coxib NSAIDs: potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med 19: 633-643.
- Feldman B, Zinkl J, Jain N (2000) Veterinary hematology. Lippincott Williams and Wilkins, Philadelphia, PP. 213.
- Gaba M, Singh D, Singh S, Sharma V, Gaba P (2010) Synthesis and pharmacological evaluation of novel 5-substituted-1-(phenylsulfonyl)-2-methylbenzimidazole derivatives as anti-inflammatory and analgesic agents. Eur J Med Chem 45: 2245-2249.
- Achar KCS, Hosamani KM, Seetharamareddy HR (2010) In vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur J Med Chem 45: 2048-2054.
- Bhattacharya S, Chaudhuri P (2008) Medical implications of benzimidazole derivatives as drugs designed for targeting DNA and DNA associated processes. Curr Med Chem 15: 1762-1777.
- Tojo J, Santamarina MT, Ubeira FM, Estevez J, Sanmartin ML ( 1992) Anthelmintic activity of benzimidazoles against Gyrodactylus sp. infecting rainbow trout oncorhynchus mykiss. Dis aquat Org 12: 185-189.
- Mohamed BG, Abdel-Alim AA, Hussein MA (2006) Synthesis of 1-acyl-2-alkylthio-,,4-triazolobenzimidazoles with antifungal, anti-inflammatory and analgesic effects. Acta Pharm 56: 31-48.
- Desai KG, Desai KR (2006) Green route for the heterocyclization of 2-mercaptobenzimidazole into ß-lactum segment derivatives containing -CONH- bridge with benzimidazole: Screening in vitro antimicrobial activity with various microorganisms. Bioorg Med Chem 14: 8271-8279.
- Hu L, Kully ML, Boykin DW, Abood N (2009) Optimization of the central linker of dicationic bis-benzimidazole anti-MRSA and anti-VRE agents. Bioorg Med Chem Lett 19: 3374-3377.
- Siakallis G, Spandidos DA, Sourvinos G (2009) Herpesviridae and novel inhibitors. Antivir Ther 14: 1051-1064.
- Refaat HM (2010) Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur J Med Chem 45: 2949-2956.
- Barril X, Beswick MC, Collier A, Drysdale MJ, Dymock BW, Fink A, et al. (2006) 4-Amino derivatives of the Hsp90 inhibitor CCT018159. Bioorg Med Chem Lett 16: 2543-2548.
- El-Sayed NS, El-Bendary ER, El-Ashry SM, El-Kerdawy MM (2010) Synthesis and antitumor activity of new sulfonamide derivatives of thiadiazolo[,2-a]pyrimidines. Europ J Med Chem 45: 1805-1820.
- Ashtawy HM, Mahapatra NR (2014) Molecular Docking for Drug Discovery: Machine-Learning Approaches for Native Pose Prediction of Protein-Ligand Complexes, in Computational Intelligence Methods for Bioinformatics and Biostatistics. Computational Intelligence Methods for Bioinformatics and Biostatistics. CIBB 2013. Lecture Notes in Computer Science, Springer, Cham, 8452: 15-32.
- Elumalai M, Muthaiah R, Alf MA (2012) Identification of curcumin targets in neuroinflammatory pathways: molecular docking scores with GSK-3β, p38 MAPK, COX, ICE and TACE enzymes. Acta Pol Pharm 69: 237-245.
- Chakraborti AK, Thilagavathi R, (2003) Computer aided Design of Non Sulphonyl COX-2 Inhibitors: An Improved Comparative Molecular Field Analysis Incorporating Additional Descriptors and Comparative Molecular Similarity Indices Analysis of ,3-Diarylisoindole Derivatives. Bioorg Med Chem 1: 3989-3996.
- Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111: 544-547.
- Vinegar R, Truax JF, Selph JL (1976) Quantitative comparison of the analgesic and anti-inflammatory activities of aspirin, phenacetin and acetaminophen in rodents. Eur J Pharmacol 37: 23-30.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences