ISSN : 2348-1927
Annals of Biological Sciences
Antifungal Defensins In Controlling Panama Wilt of Banana Fusarium oxysporum f.sp.cubense
1Donald Danforth Plant Science Center, 975 N. Warson Road, St. Louis, MO 63132, USA
2Maize Research Station, Tamil Nadu Agricultural University, Vagarai, 624613, Tamil Nadu, India
3Department of Biology, Hong Kong Baptist University, Sir Run Run Shaw Building, Ho Sin Hang Campus, Kowloon Tong, Hong
Abstract
Fusarium oxysporium f.sp. cubense causes vascular wilt and root rot in banana plantations at elevated temperatures. Fusarium oxysporium f.sp. cubense can persist in affected fields for an extended period of time on plant stubbles as macro conidia or even survive on soils as dormant chlamydospores in the absence of a suitable host plant. An in vitro antifungal assay was conducted to find the efficacy of defensin (an antifungal protein) from M. truncatula against F. oxysporium f.sp. cubense strains viz., FGSC # 8359 (Australia), FGSC # 8361 (Australia), FGSC # 8363 (MALI), FGSC # 8368 (Taiwan). In vitro antifungal assays using twofold serial dilutions of each defensin (Def1 and Def4) were carried out and Bright-field images were made using the transmitted light channel in a Zeiss LSM 510 META confocal microscope. Fungal growth inhibition was also quantitated spectrophotometrically 12 h ,24 h ,36 h ,48 h after the addition of each defensin (2 μM-20 μM). From the results, it has been postulated that the MsDef1 and MtDef4 have a biphasic effect on the membrane permeabilization of FOC depending on the defensin dose.
Keywords
Fusarium oxysporium f.sp. cubense, In vitro antifungal assays, Defensin
Introduction
Fusarium wilt of banana, commonly referred to as Panama disease, is caused by Fusarium oxysporum Schlechtend. Fr. f.sp. cubense (E.F. Sm.) W.C. Snyder and H.N. Hans [1]. This disease kills susceptible banana plants and there is no cure. The fungus infects banana plants through the roots and invades the plant’s water conducting tissues. Once FOC is introduced into banana gardens, it remains in the soil making it impossible to grow susceptible bananas in the same location for up to several decades. FOC is thought to have originated in Asia, and then spread during the 20th century to become a major problem throughout most banana production regions of the world.
Cavendish plantations have been affected with annual losses of over 75 million USD affecting family income of thousands of workers and farmers [2,3]. The global distribution of the disease has an important anthropogenic component as the infected rhizomes apparently being free of symptoms; it is not surprising that FOC got introduced into new areas with conventional plantation material [4]. FOC is thought to have originated in Asia, and then spread during the 20th century to become a major problem throughout most banana production regions of the world. An important exception is the South Pacific, where Fusarium wilt is a new disease and not yet widespread [5,6]. The fungus infects banana plants through the roots and invades the plant’s water conducting tissues. Once FOC is introduced into banana gardens, it remains in the soil making it impossible to grow susceptible bananas in the same location for up to several decades.
Plant defensins are small cysteine-rich antimicrobial proteins. Their three-dimensional structures are similar in that they consist of a-helix and three anti-parallel β-strands stabilized by four disulfide bonds. Plant defensins MsDef1 and MtDef4 are potent inhibitors of the growth of several filamentous fungi including Fusarium graminearum. MsDef1, a 45-amino acid protein from the seed of Medicago sativa, inhibits the growth of a filamentous fungus, Fusarium graminearum, at micromolar concentrations. MtDef4 is a 47-amino acid protein that is expressed constitutively and in response to biotic and abiotic stress in many tissues of a model legume, M. truncatula. Based on their effects on the morphology of fungal hyphae, antifungal plant defensins are divided into two different subgroups, referred to as morphogenic and nonmorphogenic. Morphogenic defensins inhibit hyphal growth with a concomitant increase in hyphal branching, whereas nonmorphogenic defensins inhibit hyphal growth without causing marked morphological alterations. MsDef1 is a morphogenic defensin that induces extensive hyperbranching of fungal hyphae, whereas MtDef4 is a nonmorphogenic defensin that does not induce hyperbranching [7]. MtDef4 is more potent against F. graminearum than MsDef1.
An experiment was conducted to find the efficacy of defensin (an antifungal protein) from M. truncatula against F. oxysporium f.sp. cubense, in vitro antifungal assays using twofold serial dilutions of each defensin (Def1 and Def4) were carried out and Bright-field images were made using the transmitted light channel in a Zeiss LSM 510 META confocal microscope. Fungal growth inhibition was also quantitated spectrophotometrically 12 h, 24 h, 36 h, 48 h after the addition of each defensin (2 μM-20 μM). Results are highlighted as graphical representations below.
Materials and Methods
Fusarium oxysporium f.sp. cubense cultures and maintenance strains can be found at http://www.fgcs.org, FGSC # 8359 (Australia), FGSC # 8361 Australia), FGSC # 8363 (MALI), FGSC # 8368 (Taiwan). We maintained a frozen stock of each F. oxysporum f.sp. cubense isolate in 50% glycerol at -80°C. Fusarium was thawed and grown on Czapek-Dox (CzD) plates (CzD broth with 1.5% Bacto agar, Becton Dickinson, Sparks, MD) at 22°C-28°C. Liquid cultures were inoculated from plates. The following are the Foc isolates.
FGSC # 8359: F. oxysporum f.sp. cubense; alleles: Australia; stock number: 01219; ref: phytopathology: 87:915-923.
FGSC # 8361: F. oxysporum f.sp. cubense; alleles: Australia; stock number: 8611; ref: phytopathology: 87:915-923.
FGSC # 8363: F. oxysporum f.sp. cubense; alleles: Malawi; stock number: MAL 11; ref: phytopathology: 87:915-923.
FGSC # 8368: F. oxysporum f.sp. cubense; alleles: Taiwan; stock number: F9130; ref: phytopathology: 87:915-923.
Antifungal assays
The antifungal activity of MsDef1 and MtDef2 was measured in an in vitro assay using 96-well microtiter plates. Fifty microliters of each protein dilution were added to each well of the microtiter plate containing 50 μl of spore suspension prepared in synthetic low-salt fungal medium at a concentration of 40,000 spores/ml. Fusarium oxysporium f.sp. cubense spores were harvested from CzD broth with 1.5% Bacto agar, Becton Dickinson, Sparks, MD. The fungal cultures were incubated at 24°C for 24 h. The IC50 values were determined by microscopic analysis of hyphal growth after 16 h incubation at room temperature and micro-spectrophotometrically after 48 h. Values were obtained from data collected in at least three independent experiments. Extreme hyper branching can cause denser growth and thereby higher absorbance in this assay. Growth was measured at the indicated times by determining optical density at 595 nm in a spectrophotometer (Spectra Max; Molecular Devices, Sunnyvale, CA). Optical density readings were taken after 12 h, 24 h, 36 h, and 48 h of growth at 25°C. Bright-field images were made using the transmitted light channel in a Zeiss LSM 510 META confocal microscope.
Results and Discussion
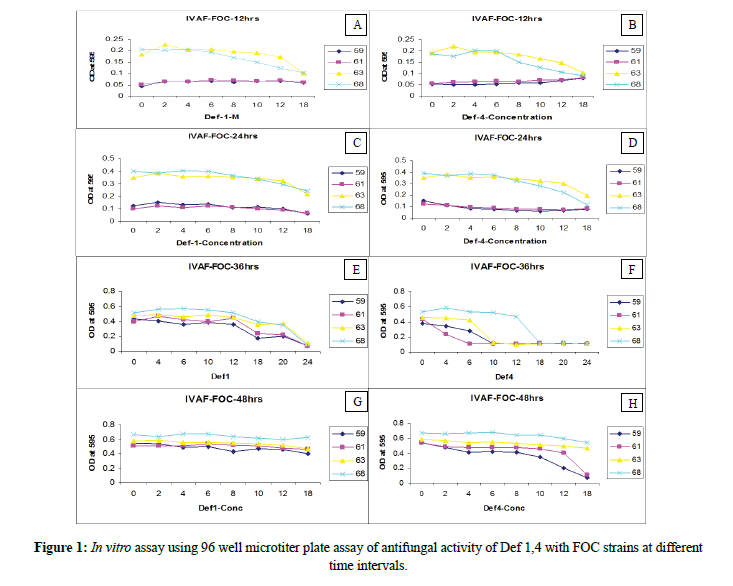
The strains FOC#8359, FOC#8361 exhibited slow growth after 12 h with less branching and minimum number of spores. The mycelium is ceonocytic, septate with micro as well macro conidia. Whereas strains FOC#8363 and FOC#8368 were able to produce well grown ceonocytic, separate branched hyphae with micro and macro conidia. Excessive branching was noticed as typical growth patterns in the above said strains under invitro conditions without adding defensins 1 and 4. Among the strains, FGSC#8359 (Australia) producing typical symptoms viz., petiole, stem necrosis, resetting of young leaves, chlorosis (data not shown) (Figure 1).
In vitro antifungal activity of Def 1, 4 with FOC strains
Defensin1
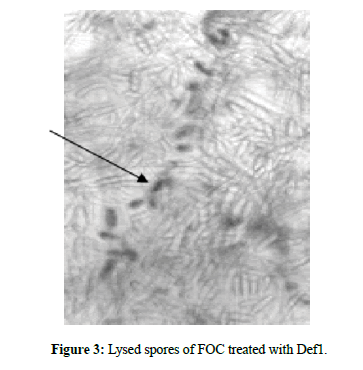
The minimal concentrations of Def1 when is inoculated with spore suspensions of the four strains showed variations viz., slightly thin mycelial cell wall with ruptured mycelium (4 μM) and less branching of hyphae in FOC#8359, FOC#8361, FOC#8363 and FOC#8368. When the concentrations raised to 6 μM FOC strains produced ruptured cell wall on the spore germlings, poorly branched, and shortened mycelia. The concentrations of 10 μM,12 μM,18 μM can be able to cause lean and lanky mycelium, broken septa, poor branching, ruptured conidial and mycelial walls (crinkled appearance) and the concentration of 18 μM produced collapsed spores, broken septa, leakage of cell contents, poor branching, very few germlings and membrane disruptions of all the four strains. The highest concentrations of Def1 (20 μM) can cause complete disruption of spores with nil germination, cell leakage, few hyphae with abnormalities in FOC#8359,61,63,68. At 24 μM caused almost no growth, completely damaged spores with turbid cell contents. All these observations were noticed within 12 h-24 h of inoculation of Def1.
Defensin4
The lowest concentrations of 4 μM, 6 μM, 10 μM showed initiation of ruptured cells and spores, poor hyphal growth. When the concentrations increased from 12 μM, 18 μM, 20 μM and 24 μM, complete death of spores with turbid cell contents was observed. Hence, it has been postulated that the lethal concentrations of 4 μM can able to control all the strains of FOC in a better way compare to defensin1. The Def1 can able to cause significant phenotypic variations with different concentrations, whereas Def4 with minimal concentrations will cause complete destruction and killing of spores under in vitro conditions (Figures 2-4).
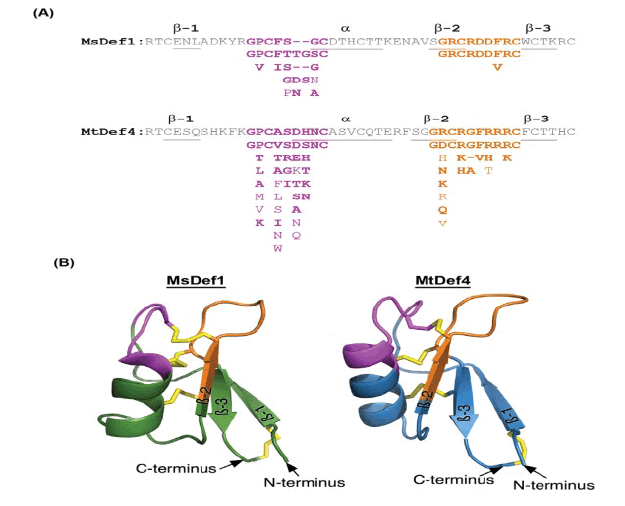
Figure 4: Highly conserved structural motifs and homology-based three-dimensional structures of plant defensins MsDef1 and MtDef4. (A) Amino acid sequences of MsDef1 and MtDef4 showing highly conserved a-core and c-core motifs. The consensus a-core (pink color) and c-core (orange color) sequences were created using 16 homologs of MsDef1 and 28 homologs of MtDef4. For both sequences, conserved amino acids of the core regions are listed underneath the respective motifs with highly conserved amino acids on the top followed by less conserved ones. α-helix and β-strands are underlined; (B) Homology-based models of MsDef1 and MtDef4. The a-core motif is indicated in pink color; the c-core motif is indicated in orange color and the four disulfide bridges are indicated in yellow color. Models were developed using the I-TASSER website for protein structure and function predictions as described in Materials and Methods [8].
From the results, it has been postulated that the MsDef1 and MtDef4 have a biphasic effect on the membrane permeabilization of FOC depending on the defensin dose. At high doses, strong permeabilization can be detected within 12 h-24 h after addition of defensin. At lower plant defensin doses, permeabilization is week. The hypothesis already proposed that the interaction of plant defensins with such a binding site subsequently enables them to insert into the plasma membrane, causing a structural disruption and alteration of the membrane’s permeability to ions such as Ca2+ and K+. In this case, the interaction involves only plant defensins and the phospholipids components of the FOC membrane.
Plant defensins (MtDf4) capable of stay at the cell surface and induce cell death through induction of a signaling cascade, was observed for MtDef4. Sagaram and colleagues identified a RGFRRR motif in the MtDef4 sequence is thought to be a translocation signal required for fungal cell entry. Other proposed mechanisms viz., receptor mediated internalization, membrane translocation (transient membrane permeabilization and lipid-assisted pore formation) and membrane permeabilization. Spelbrink et al. [9], while studying defensins from Medicago trunculata, verified that the antifungal activity of MsDef1 was due to the presence of four positively-charged amino acids, also located in the structure of the non-antifungal peptide MtDef2. Moreover, in vitro assays revealed that this region might be involved in the ability of MtDef1 to block L-type Ca++ channels in mammalian cells.
There are two major hypotheses to explain the mode of action of defensins (i) the carpet model and (ii) the pore model. Both the models, defensins are interacting with negatively charged molecules at cell membrane of pathogens, causing permeabilization leads to cell leakage and death of the cell by necrosis [10]. They will not cause any structural damage to the cell membrane, but interact with phospholipids, leading to increase in ion permeability, or transportation of peptides to intracellular environment [11,12], which enhances (ROS) Reactive Oxygen Species and activate programmed cell death. MtDef4 able to cause more physical damage of cell membranes. The γ-core sequence of MtDef4 and MsDef1 (alfAFP) showed that the high content of positively charged residues with the core of Mtdef4 could alone provide antifungal activity. Spelbrink et al. [9], was highlighted the antifungal activity of MtDef1 from Medicago trunculata was due to the presence of four positively-charged amino acids, also located in the γ-core region, might be involved in the ability of MtDef1 to block L-type Ca++ channels in mammalian cells.
Pichia pastons expressed the recombinant Nicotiana megalosiphon defensin (Nmdefo2) showed antifungal activity against F. oxysporum [13]. Residues in the Carboxy-terminal region of defensin from Medicago sativa (MsDef1) is the major determinant of antifungal activity [9]. Ramamoorthy et al. [7] described the signaling pathways to disrupt the cellular mechanism of F. graminearum. The mutants developed from F. graminearum reacted differently against MtDef1 and 4. The mutants developed by altering MAP kinase signaling cascades seemed to provide protection towards MDef1, but not to MtDef4. Both proteins inhibit the growth of FOC at micromolar concentrations with different morphological responses in the fungus. The γ-core motifs of both defensins satisfied the consensus sequence (GXCCX3-9) c and were part of the predicted three-dimensional solvent exposed β2-β3 loop region of each protein.
Application of plant defensins as antifungal agents against root pathogens, depends on understanding their structure activity relationships and Modes of Action (MOA). Current evidence suggests that antifungal plant defensins exhibit different MOA [8,14,15]. Some defensins act extracellularly on fungi and target specific cell wall/plasma membrane resident sphingolipids, disrupt membrane integrity and activate cellular toxicity pathways [14,16-18]. Present investigation evident the event of membrane permeabilization of FOC strains as reported in studies aimed at deciphering its MOA, that MtDef4 permeabilizes the plasma membrane of F. graminearum within minutes in a dose-dependent manner [7]. The spectrophotometric assays indicated that the antifungal action of Mtdef1 and 4 involves plasma membrane permeabilization, entry into fungal cells and interaction with intracellular targets as postulated by Sagaram et al. [19].
Conclusion
Plant defensins MsDef1 and MtDef4 are potential candidates for controlling Fusarium Oxysporium f.sp.Cubense (FOC) irrespective of their races. Since, the banana plantains are prone to be infected with FOC fetches low market value worldwide. Hence, using defensins as commercial fungicides or as a candidate gene for developing FOC resistant banana plants will be a boon to the banana growers in reducing the economic loss of the crop due to this major disease.
Acknowledgments
The author is thankful to Department of Science and Technology for funding through BOYSCAST programme and Dr.Yiji Xia, Dr.Dhilip Shah, Donald Danforth Plant Science Center, MO, USA-63132 for providing lab facilities, technical guidance to perform research programme.
References
- Stover, R.H. and Waite, B.H., 1954. Colonization of banana roots by Fusarium oxysporum f-cubense and other soil fungi. Phytopathology, 44, pp. 689-693.
- Masdek, N., et al., 2003. Global significance of Fusarium wilt: Asia. In 2nd International Symposium on Fusarium wilt of banana, pp. 22-26.
- Nasdir, N., 2003. Fusarium wilt race 4 in Indonesia. Research Institute for Fruits west Sumatra, Indonesia. Abstracts of Papers 2nd. International Symposium on Fusarium wilt on banana. PROMUSA-INIBAP/EMBRAPA. Salvador de Bahía, Brazil.
- Ploetz, R.C., and Pegg, K., 2000. Fusarium wilt. In: Diseases of Banana, Abaca and Enset. Jones, D.R. (Ed), CABI Publishing, Wallingford, UK, pp. 143-159.
- Davis, R.I., et al., 2000. Further records of Fusarium oxysporum f.sp. cubense from New Guinea. Australas Plant Path, 29, pp. 224-225.
- Smith, L.J., et al., 2002. Firs record of Fusarium oxysporum f.sp cubense from Yap, Federated States of Micronesia, Australas Plant Path, 31, pp. 101.
- Ramamoorthy, V., et al., 2007. Two mitogen-activated protein kinase signaling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell Microbiol, 9, pp. 1491-1506.
- Sagaram, U.S., et al., 2011. Structure-activity determinants in antifungal plant defensins MsDef1 and MtDef4 with different modes of action against Fusarium graminearum. PLoS One, 6, pp. e18550.
- Spelbrink, R.G., et al., 2004. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol, 135, pp. 2055-2067.
- Lay, F.T., et al., 2012. Dimerization of plant defensin NaD1 enhances its antifungal activity. J Biol Chem, 287, pp. 19961-19972.
- Wilmes, M., et al., 2011. Antibiotic activities of host defense peptides: more to it than lipid bilayer perturbation. Nat Prod Rep, 28, pp. 1350-1358.
- Hegedus, N., and Marx, F., 2013. Antifungal proteins: more than antimicrobials? Fungal Biol Rev, 26, pp. 132-145.
- Portieles, R., et al., 2010. NmDef02, a novel antimicrobial gene isolated from Nicotiana megalosiphon confers high-level pathogen resistance under greenhouse and field conditions. Plant Biotech J, 8, pp. 678-690.
- Anuradha, T.S., et al, 2008. Transgenic tobacco and peanut plants expressing a mustard defensin show resistance to fungal pathogens. Plant Cell Rep, 27, pp. 1777-1786.
- Wang, Y., et al., 2011. Molecular cloning and characterization of novel cathelicidin derived myeloid antimicrobial peptide from Phasianus colchicus. Dev Comp Immunol, 35, pp. 314-322.
- Di Pietro, A. et al. 2003. Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Mol Plant Pathol, 4, pp. 315-325
- Gao, A.G., et al., 2000. Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat Biotechnol, 18, pp. 1307-1310.
- Edgar, C.I., et al., 2006. Salicylic acid mediates resistance to the vascular wilt pathogen Fusarium oxysporum in the model host Arabidopsis thaliana. Australas Plant Pathol, 35, pp. 581-559.
- Sagaram, U., et al., 2013. Structural and functional studies of a phosphatidic-acid binding antifungal plant defensin MtDef4: Identification of an RGFRRR motif governing fungal cell entry. PLoS One, pp. e82485.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences