ISSN : 0976 - 8688
Der Pharmacia Sinica
Antidiabetic Activity on the Extracts of Embelia ribes in Streptozotocin Induced Diabetic Rats
Nandhakumar L1*, Kathiravan P1, Varun D1, Sambasiva Rao A1 and Nagaraja Perumal2
1Faculty of Pharmacy, Shri Indu Institute of Pharmacy, Sheriguda, Ibrahimpatnam, Telangana, India
2Faculty of Pharmacy, Karpagam College of Pharmacy, Coimbatore, India
Abstract
Several herbs with holding the antidiabetic properties are under scrutiny, by several researchers over the few decades were increasingly profound. Animals on these herbal extract studies confirmed that presence of specific components responsible for antidiabetic activity like flavonoids and polyphenolic components, which in turn also have the antioxidant activity, may be beneficial for the mankind in the management of diabetes. And hence only, in this current study, an investigation has been done on the Embelia ribes seeds for the presence of antidiabetic activity. Ethanolic extracts of seeds of Embelia ribes has been evaluated for both in vitro α-amylase activity and invivo antidiabetic activity utilizing Streptozotocin-induced diabetic wistar albino rats. The results depicts that, there was a significant and remarkable blood glucose level reduction observed in the diabetic induced groups.
Keywords
Ethanolic extract, Embelia ribes, invivo and invitro antidiabetic activity
Introduction
Blood glucose is essential to life as a regulator of homeostasis. Glucose is the obligate metabolic fuel for the brain under physiologic conditions. Glucose in plasma either comes from dietary sources or is either the result of the breakdown of glycogen in liver (glycogenolysis) or the formation of glucose in liver and kidney from other carbons compounds (precursors) such as lactate, pyruvate, amino acids, and glycerol (gluconeogenesis). Most glucose used by the body can be accounted for by six tissues [1]. The brain (45–60%), skeletal muscle (15–20%), kidney (10–15%), blood cells (5–10%), splanchnic organs (3–6%) and adipose tissue (2–4%).
The prevalence of diabetes for all age-groups worldwide was estimated to be 2.8% in 2000 and 4.4% in 2030. The total number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030 [2]. The prevalence of diabetes is higher in men than women, but there are more women with diabetes than men [3]. The urban population in developing countries is projected to double between 2000 and 2030. The most important demographic change to diabetes prevalence across the world appears to be the increase in the proportion of people >65 years of age (Table 1) [4].
| Drug class | Molecular target | Sites of action | Mode of action |
|---|---|---|---|
| Insulin Insulin lispro, Insulin aspart Insulin glargine, detemir insulin | Insulin receptor | Liver, muscle, fat | Decrease hepatic glucose production and increase peripheral glucose utilization |
| Oral Hypoglycemic Agents | |||
| Sulphonylureas Chlorpropamide Tolbutamide Glibenclamide, Glibornuride Gliclazide, Glipizide Acetohexamide, Tolazamide Meglitinides Nateglinide, Repaglinide | SU receptor/K+ ATP channel | Pancreatic β cell | Increase insulin Secretion |
| BiguanidesMetformin | Unknown | Liver (muscle) | Counter insulin Resistance |
| α-Glucosidase inhibitors -Acarbose | α-Glucosidase | Intestine | Slow carbohydrate Digestion |
| Thiazolidinediones Pioglitazone, Rosiglitazone | PPAR-γ | Fat, muscle, liver | Selectively increase insulin sensitivity |
Table 1: Actions of antidiabetic drugs used in diabetes mellitus.
Herbal treatment of diabetes
Diabetes is an important human ailment afflicting many from various walks of life in different countries. In India it is proving to be a major health problem, especially in the urban areas [5]. Though there are various approaches to reduce the ill effects of diabetes and its secondary complications, herbal formulations are preferred due to lesser side effects and low cost [6]. One of the etiologic factors implicated in the development of diabetes and its complications is the damage induced by free radicals and hence an antidiabetic compound with antioxidant properties would be more beneficial [7]. Presently, there is growing interest in herbal remedies due to the side effects associated with the oral hypoglycaemic agents for the treatment of diabetes [8]. So the traditional herbal medicines are mainly used which are obtained from plants, it plays important role in the management of diabetes mellitus [9]. In recent years, herbal medicines have started to gain importance as a source of hypoglycemic agents. Marles and Farnsworth estimated that more than 1000 plant species are being used as folk medicine for diabetes [10,11].
Combined oral therapy
In patients whose condition is not adequately controlled by diet and single-drug hypoglycemic therapy, it may be necessary to consider combination therapy [12,13].
Materials and Methods
Preparation of coarse powder and solvent fractions
The seeds were shade dried at room temperature for 10 days. Then these were milled into powder by mechanical grinder. This powder was sequentially extracted to their increasing polarity with Petroleum ether, Ethyl acetate, Ethanol respectively. About 500 gm of powdered seed was uniformly packed into a thimble in a soxhlet apparatus and fractionated with 1000 ml Petroleum ether, Ethyl acetate, Ethanol, respectively [14]. Constant heat was provided by Mantox heater for recycling of the solvent. The process of extraction continues for 1-2 hours for each solvent. The excess solvent was evaporated and the dried extracts were kept in refrigerator at 4ºC for their future use in phytochemical analysis and pharmacological screenings [15].
In vitro antidiabetic activity of Embelia ribes seed extract
Alpha-amylase inhibition assay
Chemicals or Reagents
Potato starch, trichloroacetic acid, reagents were purchased from SD Fine Pvt. Ltd., Mumbai, 3,5-dinitrosalicylic acid, Tris buffer, linoleic acid, ammonium molybdate, were purchased from Hi-Media Pvt. Ltd., Mumbai, α-amylase, α-glucosidase enzymes, xanthine oxidase, quercetin, hypoxanthine, pyrocatechol were purchased from SRL Pvt. Ltd., Mumbai. Glucose assay kit from Agappe diagnostic Pvt. Ltd., Kerala, Acarbose was obtained from Bicon Pvt. Ltd., Chennai, ferrozine, (2'2-azobis (2-amidino propane) dihydrochloride), butylatedhydroxy toluene from Loba Cheme. All other chemicals used in the study were obtained commercially and were of analytical grade.
Acute toxicity study
In a research study when a drug is administered to a biological system there will be some interactions may happen [16]. In most case these are desired and useful, but many effects are not advantageous. Acute, sub-acute and chronic toxicity studies are performed by the manufacturers in the investigation of a new drug. Acute toxicity is involved in estimation of LD50 (It is the lethal dose (causing death) to 50% of tested group animals [17].
LD50 (median lethal oral dose)
LD 50(median lethal oral dose) is a statistically derived oral dose of a substance that can be expected to cause death in percent of animals when administered by the oral route. The LD50 value is expressed in terms of weight of test substance per unit weight of animal (mg/kg)
In this study acute toxicity study was carried out in wistar albino rats. The procedure was followed by using OECD 423(Acute toxic class method). The rats are fasted overnight, prior to dosing [18]. The three dose levels are administered by the help of oral feeding needle over the prior of 24 hours [19]. After the drugs have been administered, food may be withheld for a further 3-4 hours in rats. The purpose of sighting study is to allow selection of the appropriate starting dose for main study. The test substance is administered to a single animal in a sequential manner following from the fixed dose levels of 5, 50, 300 and 2000 mg/kg [20]. The interval between dosing of each level is determined by the mortality/onset, duration and severity of toxic signs over the period of 24 hours, special attention given during the first 4 hours. Four hours after the drug administration, provide the food and water for 14 days and daily observed some parameters such as food intake, water intake, mortality, onset, duration and severity of toxic signs [21]. The animal weight is recorded on weekly once [22]. On the day fourteen all the animals are sacrificed, to isolate the organs and observe the histopathological changes. Based on the mortality result of sighting is decided and carried out with five animals per dose level (5 or 50 or 300 or 2000 mg/kg) [23]. Based on the mortality result on 14th day of observation, the doses for in vivo study are selected [24].
In vivo ant diabetic activity of Embelia ribes seed extract in streptozocin induced diabetic wistar albino rats
Wistar albino rats (150-200 grams) of both sexes were procured from College of Dr. Samsun Laboratories, Tirupur, India. Prior to the experiment the rats were housed in a clean polypropylene cages (6 rats/cages) for a period of 7 days under standard temperature (25-30), relative humidity (45–55%),dark/light cycle (12/12 hrs) [25]. The studies were performed with the approval of Organizational Animal Ethics Committee (OAEC) (ULSB/DAEC/KER/678/333/14). The animals were put in overnight fasting were deprived of food for 16 hrs but allowed free access of water.
Chemicals
Streptozocin from Loba Chemie. Standard Glibenclamide (Daonil) from Sigma Aldrich. Ethanol (Analytical grade) and 5%Dextrosesolution
Glucose Estimation Kit from Gluco Dr Super sensor
Induction of diabetes to animals
Male rats weighing 150–220 g fed with a standard diet are injected with 25 mg/kg Streptozotocin (Calbio chem) intravenously. Initially, blood glucose is increased, reaching values of 150–200 mg% after 3 h. Six–eight hrs after Streptozotocin, the serum insulin values are increased up to 4 times, resulting in a hypoglycemic phase which is followed by persistent hyperglycemia [26-28]. Severity and onset of diabetic symptoms depend on the dose of streptozotocin. After the dose of 60 mg/kg i.v., symptoms occur already after 24–48 h with hyperglycemia up to 800 mg%, glucosuria and ketonemia [29,30]. Histologically, the beta-cells are degranulated or even necrotic [31]. A steady state is reached after 10–14 days allowing using the animals for pharmacological tests [32].
Experimental design
Five groups of rats six in each groups received the following treatment schedule for 14 days.
GROUP I - Normal control (normalsaline 10 ml/kg, p.o.).
GROUP II - Streptozocin treated control (25 mg/kg, i.p.) .
GROUP III - Streptozocin (25 mg/kg, i.p.)+standard drug Glibenclamide (5 mg/kg, p.o.).
GROUP IV - Streptozocin (25 mg/kg, i.p.)+seed extract (200 mg/kg, p.o.) .
GROUP V - Streptozocin (25 mg/kg, i.p.)+seed extract (400 mg/kg, p.o.).
Plant seed extract, standard drug and normal saline were administered with the help of oral feeding needle. Group I serve as normal control which received normal saline for 14 days. Group II to Group V were diabetic control rats.
Group IV and Group V (which previously received Streptozocin 25 mg/kg) were given fixed doses of ethanol seed extract (500 mg/kg, p.o.,1000 mg/kg, p.o.) of Embelia ribes and group III received standard drug Glibenclamide (5 mg/kg, p.o.) for 14 consecutive days [33].
Collection of blood samples
Fasting blood samples were drawn from retro orbital puncture of rats at weekly intervals till the end of the study 1, 7, and 14 days [34].
Estimation of biochemical parameters Serum blood glucose
On 1, 7, and 14 days fasting blood samples were collected, serum was separated and analyzed for glucose.
Serum blood glucose
The serum blood glucose test measures the amount of glucose in the blood sample obtained from the animals. The test is usually performed to check for elevated blood glucose levels which can be an indication of diabetes or insulin inhibition.
Statistical Analysis
Statistical analysis was done by using Graph Pad Prism 6.0. All the values of Biochemical parameters and body weight were expressed as Mean ±Standard Error Mean (SEM). The values were analyzed for statistical significance using one- way analysis of variance (ANOVA), comparison was done by using Dunnett’s t-test. P values<0.05 were considered as significant, P values<0.01 were considered as very significant, P values<0.001 were considered as highly significant [35].
Results and Discussion
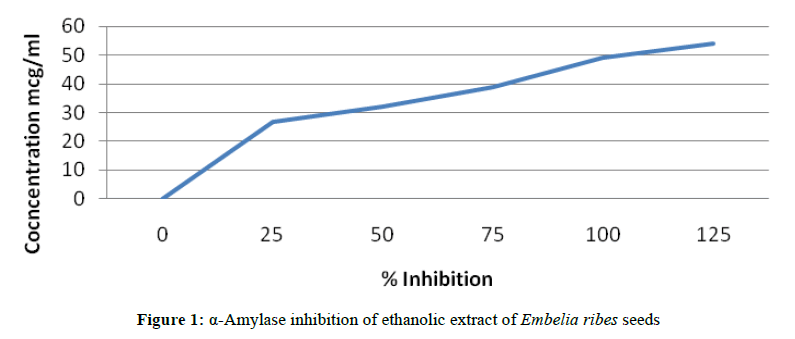
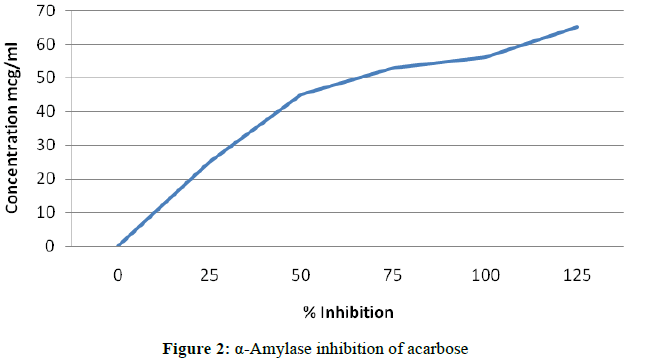
Experimental procedure for α-amylase inhibition assay
A total of 500 μl of test samples and standard drug (100-1000 μg/ml) were added to 500 μl of 0.20 mM phosphate buffer (pH 6.9) containing α-amylase (0.5 mg/ml) solution and were incubated at 25°C for 10 min (Figures 1 and 2).
Calculation of 50%inhibitory concentration (IC50)
The concentration of the plant extracts required to scavenge 50%of the radicals (IC50) was calculated by using the percentage scavenging activities at five different concentration. Percentage inhibition (I%)was calculated by
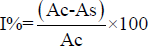 Where,
Where,
Ac = Absorbance of the control
As = Absorbance of the sample.
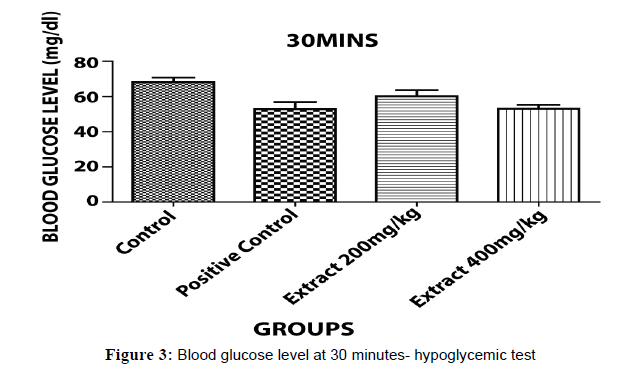
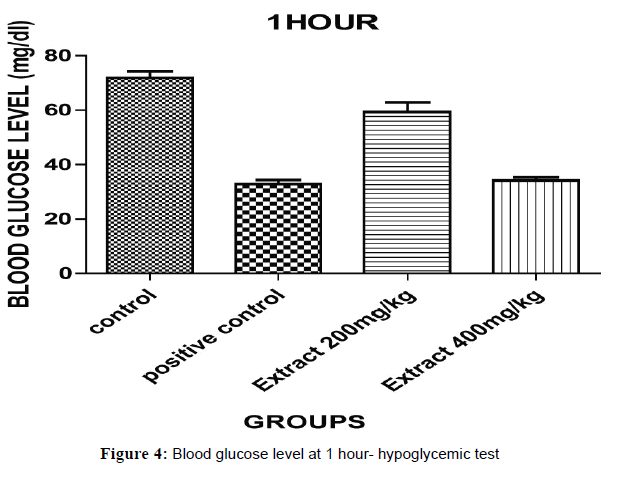
The glucose levels were analyzed by using glucometer and each value is the mean ± standard error (n= each group consist of 6 animals)(p<0.05)*, (p<0.001)**& (p<0.0001)*** as compared to control & positive control group evaluated by one way, ANOVA followed by Dunnet ‘t’ test.
The hypoglycaemic test results have been shown in Table 2 that indicates aqueous ethanolic extract of Embelia ribes treated animals 200 & 400, significantly decreased in blood glucose level (P<0.05)*, (P<0.001)**& (P<0.0001)*** when compared to control and positive (Figures 3 and 4).
| Treatment | Dose mg/kg |
Blood Glucose Level (mg/dl) | ||
|---|---|---|---|---|
| 0 min | 0.5hr | 1 hr | ||
| Control Carboxy methyl Cellulose(CMC) | 0.5 % | 68.10±1.466 | 68.00±0.300 | 70.53±0.403 |
| Positive Control Glibenclamide |
2 | 69.30±0.235 | 51.97±1.149 | 31.33±1.450 |
| Aqueous Ethanolic Extract Of Embeliaribes | 200 | 68.60±1.230 | 61.03±0.487 | 59.00±1.234 |
| AqueousEthanolic Extract Of Embeliaribes | 400 | 67.90±0.252 | 53.00±1.403 | 34.17±1.145 |
Table 2: Hypoglycemic test
In vivo antidiabetic study
The experimental results have been indicated in Table 3. The negative control group glucose levels were significantly increased when compared to each other groups. All the groups of animals were affected in diabetes, which indicated blood glucose levels were slight changes in the blood glucose level for normal control group at 7th and 14th days. On day 7th glucose levels were significantly decreased Glibenclamide 2 mg/kg treated group (P<0.05)*, (P<0.001)** and (P<0.0001)*** when compared with control group at 7th and 14th days. The ethanolic seed extract of Embelia ribes treated groups 200 and 400 mg/kg were dose dependent manner decreased (P<0.001)** and (P<0.0001)*** when compared with control group but positive control have more anti diabetic activity at 7th day. The aqueous ethanolic seed extract of Embelia ribes at the dose level 400 mg/kg have equipotent activity when compared with positive control at 7th day. The ethanolic seed extract of Embelia ribes 200 and 400 mg/kg have been expressed dose dependent anti diabetic action (P<0.001) ** and (P<0.0001) *** when compared to control and positive control. On day 14th, ethanolic leaf extract of Embelia ribes treated animals 200 and 400 mg/kg significantly decreased and maintain the blood glucose level.
| Sl. No. | Treatment | Blood glucose level (mg/dl) day | ||
|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | ||
| 1 | Normal control 10 ml/kg P.O |
78.3±0.734 | 76.4±1.045 | 75.1± 0.342 |
| 2 | Negative control 100 mg/kg I.P |
261.0±0.960 | 271.3±1.800 | 274.0±1.300 |
| 3 | Positive control (Glibenclamide 2mg/kg) P.O |
252.6±0.340 | 131.6±1.900 | 113.0±1.420 |
| 4 | EEER 200 mg/kg P.O | 262.0±2.220 | 251.2±1.123 | 244.1±3.256 |
| 5 | EEER 400 mg/kg P.O | 263.0±1.36 | 175.0±0.470 | 163.1±0.701 |
EEER -Ethanolic extract of Embelia Ribes seed (The values were expressed as Mean ± S.E.M. (n=6 animals in each group).
Table 3: Results of the effects of ethanolic extract on blood glucose levels
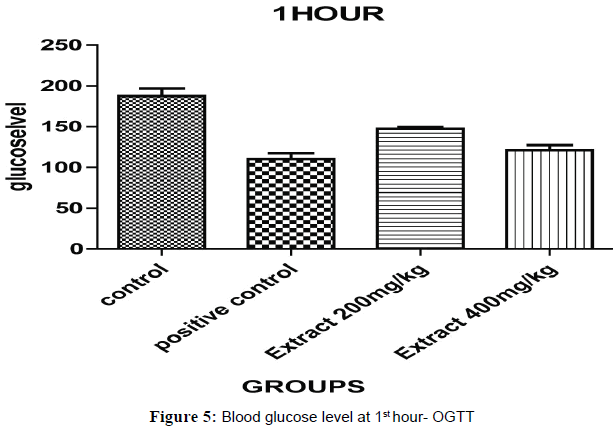
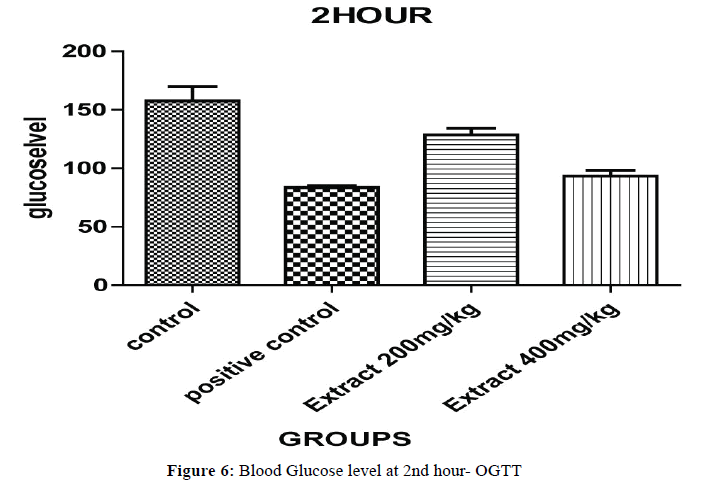
Oral Glucose Tolerance Test (OGTT) results have been expressed on Half hour after the glucose treatment, all the groups of animal blood glucose levels were significantly increased (Figures 5 and 6). The blood glucose levels were significantly decreased for, aqueous ethanolic extract of Embelia ribes 200 and 400 mg/kg (P<0.001) ** and (P<0.0001) ** when compared to control and positive control at 1 hour and each and every ½ hour blood glucose levels (P<0.05)*, (P<0.001)** and (P<0.0001)*** were changes in the dose dependent manner extract treated group of animals compared to control and positive control but 400 mg/kg (Table 4).
| Treatment | Dose mg/kg |
Blood Glucose Level (mg/dl) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 0.5hr | 1 hr | 1.5hr | 2 hr | 2.5hr | 3hr | ||
| Control Carboxymethyl Cellulose(CMC) | 0.5% | 68.20±1.40 | 141.20±4.26 | 187.40±7.45 | 172.60±1.45 | 156.00±13.67 | 152.80±1.34 | 130.20±1.68 |
| Positive Control Glibenclamide |
2 | 69.06±0.65 | 104.25±6.35 | 110.40±1.94 | 93.63±1.32 | 83.02±1.34 | 77.15±4.26 | 74.28±2.47 |
| Ethanolic Extract of EmbeliaRibes | 200 | 68.63±1.30 | 128.20±6.09 | 147.10±1.40 | 138.40±2.64 | 128.30±4.63 | 113.60±6.45 | 108.70±5.15 |
| Methanolic Extract of EmbeliaRibes | 400 | 68.34±1.43 | 115.10±5.14 | 121.10±6.36 | 103.40±0.46 | 93.36±4.87 | 86.67±3.23 | 83.67±1.56 |
The glucose levels were analyzed by using glucometer and all values are expressed as Mean ± SEM (n=6), Group 2 was compared with group 1, Groups–3,4 were compared with group 2; *p<0.05, **p<0.01, p<0.001*** evaluated by one way, ANOVA followed by Dunnet ‘t’ test.
Table 4: Oral glucose tolerance test
Conclusion
At this juncture, in this study, the ethanolic extracts of Embelia ribes utilized for the investigation of antidiabetic activity has been considerably significant and remarkable, as compared with the standard drug. Hence the seeds of Embelia ribes have the potential benefit of controlling the blood glucose level in the selected animal model. Hence it may be a viable and conducive option, if the Embelia ribes may be considered to for the antidiabetic activity.
References
- Barbara, G., Joseph, T.,Pharmacotherapy Handbook. 7thEdn.,2009.
- Lippincott, W.,Introductory Clinical Pharmacology, 2000. 7thEdn., p. 487-488.
- Charles, R., Robert, E., Modern Pharmacology with Clinical Applications, 2012.5thEdn., p. 767-768.
- Fronzo, D.,Diabetes, 2009. 58(1): p. 773-795.
- Modak, M., Dixit, P., Londhe, J., et al., Journal of Clinical Biochemistry and Nutrition,2007. 40(3): p. 163-173.
- Sabitha, V., Ramachandran, S., Naveen, K., et al., Journal of Pharmacy and Bioallied Science, 2011. 3(3): p. 397-402.
- Shukla, A., Bukhariya, V., Mehta, J.,et al., International Journal of Biomedical and Advance Research, 2011. 2(1): p. 57-58.
- Li, W., Zheng, H., Bukuru, J.,et al., Journal of Ethnopharmacology, 2004. 92(1):p. 1-21.
- Sy, C., Cisse, A., Nongonierma, R.,et al., Journal of Ethnopharmacology,2005. 98(2):p. 171-175.
- Salim, B., International Journalof Diabetes & Metabolism, 2005. 13(1): 111-134.
- Zia, T., Hasnain, S., Hasan, S.,Journal of Ethnopharmacology, 2001. 75(1): p. 191-195.
- Heilbronn, L., Smith, S., Ravussin, E., Obesity, 2004. 28(3):p. 12-21.
- Verma, M., Singh, H., Satyanarayana, M.,et al., Journal of Medicinal Chemistry, 2012. 55(10):p. 4551-4567.
- Raj, K., Sripal, R., Chaluvadi, M.,et al., Indian Journal of Pharmacology, 2001. 33(1):p. 2-16.
- Mohan, S., Nandhakumar, L., Senthilkumar, C., et al.,International Journal of Pharmaceutical Research& Allied Science, 2013. 2(2):p. 1-8.
- Tapas, A., Sakarkar, D., Kakde, R.,Tropical Journal of Pharmaceutical Research, 2008. 7(3): p. 1089-1099.
- Thaipong, K., Boonprakob, U., Crosby, K., et al., Journal of Food Composition and Analysis, 2006.19(1): p. 669-675.
- Kaushik, P., Kaushik, D., Lal, S., Journal of Pharmaceutical Education and Research, 2011. 2(1):p. 50-53.
- Krutika,T., LeenaP., Dnyanesh, L., et al., International Journal of Research in Pharmaceutical and Biomedical Sciences, 2012. 3(2):p. 730-733.
- Ramakrishna, D., Vidyasagar, G., Pavan, K., et al., International Journal of Pharmaceutical Sciences and Research, 2011. 2(3):p. 98-110.
- Bnouham, M., Ziyyat, A., Mekhfi, H.,et al., International Journal of Diabetes Metabolism, 2006. 14(1):p. 1-25.
- Aissaoui, A., Zizi, S., Israili, H., et al.,Journal of Ethnopharmacology, 2011. 137(4):p. 625-661.
- Calderon, J., Burgos, E., Perez, C.,et al., Mini Review in Medicinal Chemistry, 2011. 11(4):p. 298-344.
- Habtemariam, S., Natural Product Communications, 2011. 6(2):p. 201-203.
- Koyama, E., Sakai, N., Ohori, Y.,et al., Food and Chemical Toxicology, 2003. 41(1): p. 875-883.
- Amidon, G., Lennernas, H., Shah, V.,et al., Pharmaceutical Research, 1995. 12(3):p. 413-420.
- Ming, J., Hui, Y., Yan,W., et al.,African Journal of Pharmacy and Pharmacology, 2011. 5(18):p. 2079-2085.
- Krutika, T., Leena,P., Dyanesh,L.,et al., International Journal of Research in Pharmaceutical and Biomedical Sciences, 2011.3(2): p. 730-733.
- Yuichi, T., Masaaki, I., Hiromasa,U., et al., European Journal of Pharmaceutical and Biopharmceutics, 2010.79(12): p. 559-565.
- Senthilkumar, K., Poluru,R., Sathish,S., International Journal of Pharmacyand Biological Sciences, 2011.2(2): p. 63-69.
- Ghada, A.,Hossam, A., Mohamed, M., et al.,ZeitschriftfürNaturforschung, 2008. 63(10): p. 658-662.
- Nidhi, S., VeenaG., Indian Journal of Biochemistry and Biophysics, 2012. 49(1): p. 55-62.
- Daniel, H., Nicoleta, H.,Geza, B., et al.,Food chemistry,2012. 132(3):p. 1651-1659.
- Mamdouh, A.,Monira, A.,Abd, K., Naturforsch,2011. 59(1):p. 726-733.
- Fang, X., Gao, J., Zhu, D.,Life Sciences,2014. 82(1): p. 615-622.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences