ISSN : 2348-9502
American Journal of Ethnomedicine
Antibacterial Activities of the Leaf and Bark Extract of Persea americana
Department of Microbiology, Federal University of Technology, P.M.B. 704, Akure, Nigeria
Abstract
The antibacterial activities of the methanolic leaf and bark extract of Persea americana was tested in vitro on bacterial isolates namely: Streptococcus pyogenes, Proteus mirabilis, Salmonella typhi, Klebsiella pneumoniae, Escherichia coli, Bacillus subtilis (NCIB 3610), Staphylococcus aureus (NCIB 8588), Escherichia coli (NCIB 86), Klebsiella pneumoniae (NCIB 418) and Pseudomonas aeruginosa (NCIB 950), using the agar well diffusion method. The leaf extract was able to inhibit the growth of the test organisms at a concentration of 50.0mg/ml except Escherichia coli, Salmonella typhi, Proteus mirabilis and Escherichia coli (NCIB 86), while the bark extract at the same concentration inhibited the test organisms except Salmonella typhi, Escherichia coli, Bacillus subtilis (NCIB 3610) and Escherichia coli (NCIB 86). The highest zone of inhibition of 6.0 mm and 12.0 mm was observed on Staphylococcus aureus (NCIB 8588) for the leaf and bark extract respectively, while the least zone of inhibition was observed on Klebsiella pneumoniae (2.0 mm) for leaf extract and Proteus mirabilis (3.0 mm) for bark extract. The Minimum Inhibitory Concentration (MIC) of the leaf extract ranged from 10.0 to 30.0 mg/ml and 5.0 to 30.0 mg/ml for the bark extract. The antibacterial activity of the methanolic plant extract inhibited the growth of the test isolates as much as that of the commercial antibiotics. There was a decrease in the bacterial count as the exposure time to
Keywords
Antibacterial, Zone of inhibition, Agar well diffusion, Phytochemicals and Rate of killing.
INTRODUCTION
Plants have been described as gift of nature; they have been used as a therapeutic agent against various infectious diseases affecting both human and animals [1]. As such, much emphasis has been placed on the exploitation of medicinal plants that can be used in the treatment of infectious diseases [2]. The use of medicinal plants in folk medicine still serve as an alternative means of cost effective treatment of infections in covering the basic health needs of people in developing countries. The secondary metabolites (bio active compounds) produced by these plants have been linked to their high medicinal potency and as such enable them to be used as a source of raw materials in the exploration of antimicrobial agents in the industry. Various plant parts, including herbs, spices, fruits, vegetables and tropical plants have been showed to contain these natural antimicrobials which are of intense medicinal benefits [3]. As more and more habitats of rich biodiversity are threatened by the forces of development, scientists all over the world are scrambling to identify new plant species and to learn about their traditional uses before they are lost forever4.
Persia americana (family Lauraceae) is a tree plant known as ‘avocado’, ‘avocado pear’ or ‘alligator pear’. They are widely cultivated throughout the tropics and subtropics of the world for their edible fruits and for some economic and therapeutic uses [5]. Avocado pears are rich source of soluble phenolics, ascorbic acid and betalains compared to most common fruits and vegetables [6]. It is recommended for gastritis, gastroduodenal ulcer, hypertension, anaemia and exhaustion [7]. Previous studies by Adeboye et al [8], Adeyemi et al [9] and Gomez-Flores et al [10] have shown the pharmacological activity of Persea americana.
This work is carried out to assay for the effect of the leaf and bark extract of Persea americana on some pathogenic bacteria so as to be used traditionally as a source of therapeutic agent.
MATERIALS AND METHODS
Collection and preparation of leaf and bark of Persea americana
The leaves and bark cuttings were collected from the forest and wild life reserve of the Federal University of Technology, Akure, Nigeria, where they were found to be growing naturally. The bark samples were cleaned of epiphytes and necrotic parts were removed in running water. The leaves and bark cuttings were dried in an oven at a temperature of 40°C for 5 days. The dried plant parts were separately crushed into fine powder using milling machine. About 600 g of the pulverized plant parts were each weighed and soaked in methanol to saturation for a period of 72 hours. The mixtures were agitated after the addition of the solvent. They were then sieved with muslin cloth and filtered using number 1 Whatmann filter paper. The filterates were collected in separate beaker and dried invacuo using rotary evaporator (Resona, Germany).
Test isolates
The typed cultures: Bacillus subtilis (NCIB 3610), Staphylococcus aureus (NCIB 8588), Escherichia coli (NCIB 86), Klebsiella pneumoniae (NCIB 418) and Pseudomonas aeruginosa (NCIB 950) were obtained from the stock culture of the Department of Microbiology, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Osun State, Nigeria, while clinical isolates: Streptococcus pyogenes, Proteus mirabilis, Salmonella typhi, Klebsiella pneumoniae and Escherichia coli were collected at the Don Bosco Catholic Medical Centre, Araromi Street, Ondo State, Nigeria. All the bacterial species used were maintained on nutrients agar slants and stored in the refrigerator at a temperature of 4°C from where they were subcultured unto fresh media at regular intervals.
Standardization of the test isolates
About 0.2 ml of a 24 hours old broth culture of the test isolates was dispensed into 20 ml sterile nutrient broth and incubated for 3-5 hours to obtain 0.5 McFarland standards (106 cfu/ml) according to the method of Oyeleke and Manga [11].
Antibacterial activities of the leaf and bark extract of Persea americana
This was done using the agar well diffusion method as described by Olutiola et al [12]. About 50.0 mg/ml of both extract were prepared with 30% Dimethyl Sulphoxide used as re-constituting solvent. The plates were incubated at 37°C for 24 hours. Areas showing clear zone around bored holes indicates inhibition of the organisms by the extracts and these were measured and recorded in mm.
Minimum Inhibitory Concentration of leaf and bark extract of Persea Americana
Five concentrations (50.0 mg/ml, 30.0 mg/ml, 20.0 mg/ml, 10.0 mg/ml and 5.0 mg/ml) of the methanol leaf and bark extracts were prepared and assayed according to the method of Doughari et al [13]. The plates were incubated at 370C for 24 hours. Concentration of the extract below where there was no inhibition was recorded as the Minimum Inhibitory Concentration (MIC).
Phytochemical screening
The leaf and bark extract was screened for terpenoids, tannins, saponins, flavonoids and cardiac glycoside as described by Trease and Evans [14].
Antibiotics sensitivity test
The disc diffusion method as described as Khan et al [15] was used to determine the antibacterial activities of standard commercially produced antibiotics against the test isolates.
Rate of killing of the organism by the extract
About 5 ml of 50 mg/ml of the methanol leaf and bark extracts and 5 ml of the standard culture was added together in a sterile test tube and allowed to stand for about 24 hours. At intervals of one hour, about 1 ml of the mixture was pour plated on nutrient agar medium and incubated at 37°C for 24 hours, according to the method of Ogundare and Akinyemi [16]. The microbial load was thereafter determined.
RESULTS AND DISCUSSION
The emergence of drug resistance human pathogenic organisms against the currently used chemotherapeutic agents is on the increase and requires global attention. Researches on the use of plants as antimicrobials will not only authenticate their use in traditional medicine but will provide future promises in the discovery of new drugs with antimicrobial potential.
Table 1 shows the antibacterial activity of the methanol extract of Persea americana, the highest inhibitory effect to the leaf and bark extract was observed on Staphylococcus aureus NCIB8588 with a zone of inhibition of 6.0 mm and 12.0 mm respectively, while Klebsiella pneumoniae (both typed and clinical isolates) were the least inhibited by the leaf extract with a zone of inhibition of 2.0 mm and Proteus mirabilis with a zone of inhibition of 3.0 mm by the bark extract. However, both extract had no inhibitory effect on Salmpnella typhi and Escherichia coli (typed and clinical isolates). Decoctions and extracts of the various plant parts of Persia americana are reputed for their use in the treatment of infections of microbial origin in Nigeria and other African countries [17]. Clinical isolates are known to carry a resistance gene which makes them not susceptible to antimicrobial agents [18].
Table 1: Antibacterial activity of the methanol leaf and bark extract of Persea Americana
| Organisms | Zone of inhibition (mm) at the concentration of 50 mg/ml | |
|---|---|---|
| Leaf extract | Bark extract | |
| Streptococcus pyogenes | 4.0 | 8.0 |
| Proteus mirabilis | - | 3.0 |
| Salmonella typhi | - | - |
| Klebsiella pneumoniae | 2.0 | 5.0 |
| Escherichia coli | - | - |
| Bacillus subtilis (NCIB 3610) | 4.0 | - |
| Staphylococcus aureus (NCIB 8588) | 6.0 | 12.0 |
| Escherichia coli (NCIB 86) | - | - |
| Klebsiella pneumoniae (NCIB 418) | 2.0 | 8.0 |
| Pseudomonas aeruginosa (NCIB 950) | 4.0 | 4.0 |
The inhibitory effect observed on Staphylococcus aureus and Pseudomonas aeruginosa indicates that the plant might possess some wound healing property if further purified. However, the bark extract of Persea americana might also be used in treating urinary tract infection caused by Proteus sp due to its inhibitory effect on the organism. The clinical isolates were more resistant to the extract than the typed isolates; this might be due to the indiscriminate exposure of the clinical isolates to antibiotics which has generated resistance [19].
The minimum inhibitory concentration (MIC) assay of the plant extract on Table 2 revealed that the least inhibitory effect was exhibited both on Staphylococcus aureus (NCIB 8588) at 10.0 mg/ml for the leaf extract and 5.0 mg/ml for the bark extract. This low MIC showed a strong antibacterial effect on the test organisms, particularly the bark extract of Persea americana.
Table 2: Minimum Inhibitory Concentration of the methanol leaf and bark extract of Persea americana
| Organism | Concentration of extract (mg/ml) | |
|---|---|---|
| Leaf extract | Bark extract | |
| Streptococcus pyogenes | 30.0 | 10.0 |
| Proteus mirabilis | ND | 30.0 |
| Klebsiella pneumonia | 30.0 | 20.0 |
| Bacillus subtilis (NCIB 3610) | 20.0 | ND |
| Staphylococcus aureus (NCIB 8588) | 10.0 | 5.0 |
| Klebsiella pneumoniae (NCIB 418) | 30.0 | 10.0 |
| Pseudomonas aeruginosa (NCIB 950) | 30.0 | 30.0 |
Key: ND – Not Determined
Phytochemical screening of the plant extracts in Table 3 showed that secondary metabolites like saponins, tannins, flavonoids and terpenoids were found to be common to both leaf and bark extract. However, only alkaloid was completely absent in both extract. These bioactive components might be responsible for the antibacterial activity of the extracts [20].
Table 3: Phytochemical constituents of the methanol leaf and bark extract of Persea americana
| Phytochemical groups | Leaf extract | Bark extract |
|---|---|---|
| presence/absence | presence/absence | |
| Saponins | +ve | +ve |
| Tannins | +ve | +ve |
| Phleobatannin | +ve | -ve |
| Alkaloids | -ve | -ve |
| Anthraquinone | +ve | -ve |
| Flavonoids | +ve | +ve |
| Terpenoids | +ve | +ve |
| Cardiac glucoside | ||
| Legals Test | +ve | +ve |
| Salkowski Test | +ve | +ve |
| Keller Killian Test | +ve | +ve |
| Liebermans Test | +ve | +ve |
Keys: + = presence, - = absence
The demonstration of antibiotic sensitivity test against both Gram-positive and Gram-negative bacteria as shown on Table 4 indicates the broad spectrum activity of gentamycin as compared with the other antibiotics. Salmonella typhi and both clinical and typed isolates of Escherichia coli were susceptible to one or more antibiotics. However, these bacteria were resistant to the plant extract. The high inhibition values recorded by the antibiotics than the plant extracts may be due to its purified nature, as reported by Doughari et al [13], that antibiotics are in a refined state while plant extracts are still in crude state.
Table 4: Antibiotic sensitivity test on bacterial isolates
| Gram positive organisms | Zones of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| AUG | GEN | COT | STR | TET | CHL | ERY | CXC | |
| Streptococcus pyogenes | - | 8.0 | - | 13.0 | 10.0 | 14.0 | 9.0 | - |
| Klebsiella pneumonia | - | 10.0 | - | 6.0 | 6.0 | 5.0 | 16.0 | - |
| Bacillus subtilis (NCIB 3610) | 2.0 | 3.0 | - | 3.0 | - | - | - | - |
| Staphylococcus aureus (NCIB 8588) | - | 4.0 | 11.0 | 15.0 | - | 4.0 | 15.0 | - |
| Klebsiella pneumoniae (NCIB 418) | - | - | - | - | - | - | - | - |
| Gram negative organisms | AMX | AUG | COT | GEN | NAL | NIT | OFL | TET |
| Proteus mirabilis | - | - | 10.0 | 17.0 | - | - | - | 10.0 |
| Salmonella typhi | 3.0 | - | - | - | - | - | - | 3.0 |
| Escherichia coli | 6.0 | 4.0 | - | 11.0 | 13.0 | 15.0 | 17.0 | 12.0 |
| Pseudomonas aeruginosa (NCIB 950) | - | - | 3.0 | 2.0 | - | 11.0 | 2.0 | - |
| Escherichia coli (NCIB 86) | - | 2.0 | - | 3.0 | 11.0 | - | 12.0 | 1.0 |
Keys: GEN – Gentamycin (10 μg), STR – Streptomycin (10 μg), TET – Tetracycline (10 μg), AMX – Amoxycillin (30 μg), CHL – Chloramphenicol (10 μg), CXC – Coxacillin (5 μg), ERY – Erythromycin (5 μg), NAL – Nalidixic acid (30 μg), NIT – Nitrofurantion (20 μg), COT – Cotrimazole (25 μg), AUG – Augmentin (30 μg), OFL – Oflaxacin (5 μg), (-) = no inhibition.
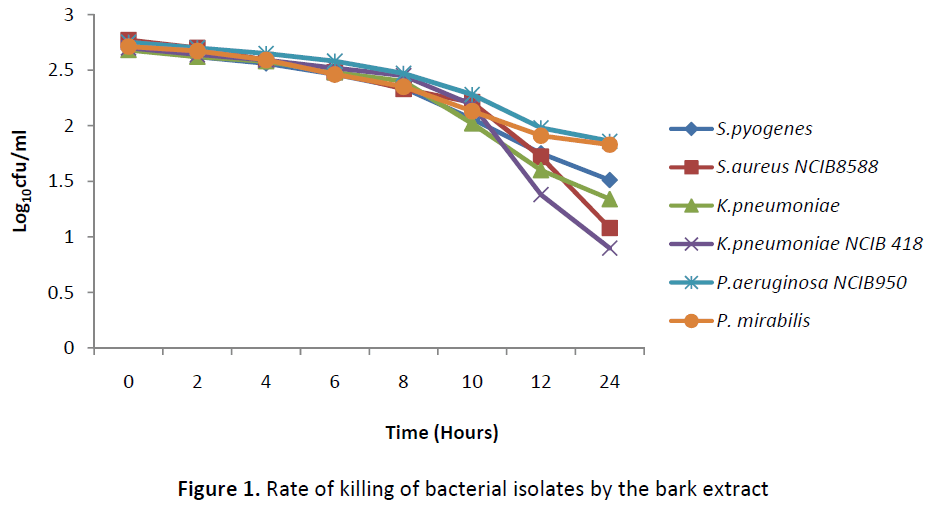
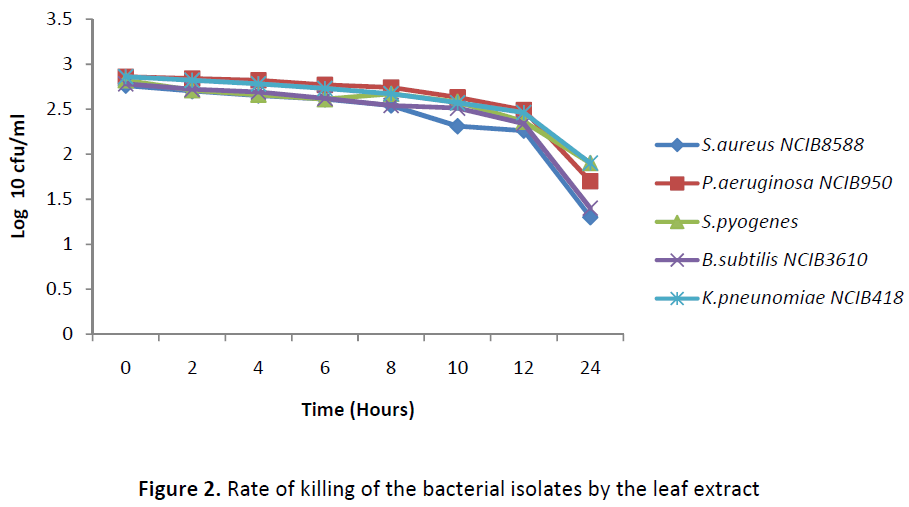
The result of the rate of killing of the organism in Figures 1 and 2 shows a gradual reduction in the number of colonies from 0 hour to 24 hours in all the test isolates, however at 24 hours, there was no total inhibition of any of the isolates. An increase in the exposure time beyond 24 hours might cause a total cidal effect on the organisms or an increase in the concentration of the extracts.
CONCLUSION
The bark extract of Persea americana exhibited a much better antibacterial effect on the test isolates than the leaf extract. The availability and accessibility to plant parts makes the use of Persea americana a cost effective alternative medicine to the commercial antibiotics to which most organisms are now developing resistance. Further purification of the extract and identification of the active component is necessary to enhance greater antibacterial potency. Herbal medicine has proven to be of great importance to the treatment of basic human diseases from time immemorial, this natural endowment (plants) should be exploited scientifically so as to tackle health related issues.
ACKNOWLEDGEMENT
Authors are thankful to Microbiology Laboratory, Obafemi Awolowo University Teaching Hospital complex, Ile-Ife, Osun State and Don Bosco Catholic Medical Centre, Araromi Street, Ondo State, Nigeria for providing the test isolates.
REFERENCES
- Beegum NR, Devis GT. Antibacterial activity of selected seaweeds from kovalam South West Coast of India. Asian J. Microbiol. Biotech. Env. Sci. 2003; 5(1):319-322.
- Cox PS. Bioactive Compound from Plants: Ciba Foundation Symposium 154. John Wiley and Sons Ltd, London. 1990. p. 40- 41.
- Ciocan ID, Bara II. Plant products as antimicrobial agents. Genetică și Biologie Moleculară. 2007; 8(1):151‐156.
- Morgenstern K. Plants as gateways to the sacred. Sacred Earth newsletter. pleasantville, New york. 2003.
- Purseglove JW. Tropical Crops: Dicotyledons. 3rd ed. Longman Group Ltd, New York. 1977. p. 41-42.
- Garcia-Alvarado JS, Verde-Star MJ, Heredia NL. Traditional uses and scientific knowledge of medicinal plants from Mexico and Central America. J. Herbs, Spices Med. Plants. 2001; 8:37-90
- Pamplona-Roger GD. Encyclopedia of Medical Plant. In: Gelabert F, Carmona R, Gonzalez P, editors. Encycloedia of Medicinal plants. Graficas Reunidas, Madrid, Spain. 1999. p. 795.
- Adeboye JO, Fajonyomi, MO, Makinde JM, Taiwo OB. A preliminary study on the hypotensive activity of Persea Americana leaf extracts in anaesthetized normotensive rats. Fitoterapia. 1999; 70:15-20.
- Adeyemi OO, Okpo SO, Ogunti OO. Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill (Lauraceae). Fitoterapia. 2002; 73:375-380.
- Gomez-Flores R, Verastegui-Rodriguez L, Quintanilla-Licea R, Tamez-Guerra P, Tamez-Guerra R, Rodriguez-Padilla C. In vitro rat lymphocyte proliferation induced by Ocinum basilicum, Persea americana, Plantago virginica and Rosa spp. extracts. J. Med. Plant Res, 2008; 2:5-10.
- Oyeleke SB, Manga SB. Essentials of Laboratory Practical in Microbiology. Tobest Publisher, Minna, Nigeria. 2008. p. 36-75.
- Olutiola PO, Famurewa O, Sonntag HG. An Introduction to General Microbiology, A practical approach. 2nd ed. Bolabay Publications, Ikeja. Lagos, Nigeria. 2000. p. 35-66.
- Doughari JH, Pukuma MS, De N. Antibacterial effects of Balanites aegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella typhi. Afr. J. Biotechnol. 2007; 6(19):2212-2215.
- Trease GE, Evans WC. Pharmacognosy. 15th ed. B Saunders, London. 2002. p. 137- 440.
- Khan MR, Kihara M, Omotosho AD. Antibacterial and antifungal activities of Barrington asiatica. Fitoterapia. 2002; 5:255-260.
- Ogundare AO, Akinyemi AI. Phytochemical and antibacterial properties of Combretum mucronatum (Schumach) leaf extract. Afr. J. Microbiol. Res, 2011; 5(18):2632-2637.
- Okigbo RN, Mbajiuka C, Njoku CO. Antimicrobial potentials of (UDA) Xylopia aethopica and Occimum gratissimum L. on some pathogens of man. Int. J. Mol. Med. Adv. Sci, 2005; 1(4):392-397.
- Adeleye IA, Ogunniyi AA, Omonigbehin EA. Antimicrobial activity of some local herbs on common skin pathogens. Bio Sci. Res. Commun. 2003; 15(3):231-239.
- Oladunmoye MK. Comparative studies on the antimicrobial activities of leaf extracts from six cassia species. PhD thesis. Federal University of Technology, Akure, Nigeria, Department of Microbiology; 2005.
- Igbinosa OO, Igbinosa EO, Aiyegoro OA. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn). Afr. J. Pharm. Pharmacol, 2009; 3(2):058-062.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences