ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
An Optimized Culture System of Haploid Plants from Unfertilized Ovules of Male-Sterile Tobacco
Muna Alariqi1,3, Chen Luo1, Cao Jinglin2* and Jin Shuangxia1
1Department of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan, China
2Tobacco Research Institute of Hubei Province, Wuhan, Hubei, China
3Department of Crops Science and Genetic Improvement, Food and Environment, Sana’a University, Sana’a, Yemen
- *Corresponding Author:
- Cao Jinglin
Tobacco Research Institute of Hubei Province, Wuhan,
China,
E-mail: caojinglin670425@sohu.com
Received date: November 04, 2021, Manuscript No. AJPSKY-22-11428; Editor Assigned date: June 17, 2022, PreQC No. AJPSKY-22-11428(PQ); Reviewed date: June 28, 2022, QC No. AJPSKY-22-11428; Revised date: July 08, 2022, Manuscript No. AJPSKY-22-11428(R); Published date: July 15, 2022, DOI: 10.36648/2249- 7412.12.7.303
Citation: Alariqi M, Luo C, Jinglin C, Shuangxia J(2022) An Optimized Culture System of Haploid Plants from Unfertilized Ovules of Male-Sterile Tobacco. Asian J Plant Sci Res Vol:12 No:7.
Abstract
Haploid breeding is an important method for efficient modern breeding of crops. Compared to the natural doubling, doubling induction of tobacco anther derived haploids often leads to the deterioration of yield and quality, limited utilization value and the doubling low frequency. Obtaining lines similar to the conventional selfing with no deterioration of yield and quality is not an easy task. Therefore, in view of that, the haploid induction of unfertilized ovule in vitro was studied using the tobacco male-sterile hybrid KRK26. The ovules of five developmental stages were selected and cultured on five optimized media based on H, HW and N6. The results showed that the more mature the embryo sac was, the worse the induction effect of ovule proliferation was under the test scope. The ovules which developed earlier, i.e. the corolla of the flower was about to be exposed from the calyx stage to the time when the corolla was twice as long as the calyx, are recommended to be used for culture. Among the five optimized media, the medium CM1 based on H medium had the best induction effect on embryoids, and adjusting the agar concentration to 1% on the basis of CM1 was beneficial to inhibit the vitrification of embryoids. The embryoids were transferred to H medium without hormones, which could avoid the browning of embryoids, and facilitate the induction of regenerated buds. The regenerated buds were transferred to H medium or N6 medium supplemented with 10 mg/L IAA, which was beneficial to the rapid rooting of regenerated buds and obtaining a large number of regenerated plants. The haploid regenerated plants were identified based on Flow cytometry test. In this study, an optimized culture system was established to induce haploid plants from unfertilized ovule of male sterile tobacco.
Keywords
Tobacco; Male sterility; Unfertilized ovule; Haploid; Regenerated plant.
Introduction
In recent years, haploid breeding has been widely used in crops breeding. As an important part of modern efficient breeding system, haploid breeding has significant advantages including shortening breeding years and improving selection efficiency [1-3]. In general, haploid can be produced by culturing anther or microspore in vitro, haploid originated from anther is known as male haploid. This technique is widely used in breeding system of vegetable crops such as Chinese cabbage and cauliflower, however, the microspore culture of Solanaceous crops such as eggplant [4] and potato [5] is challenging and most experiments are still in the stage of callus or embryoid. Tobacco, which is also a solanaceous crop, is an important economic crop in the world. In the middle and late 20th century, the American tobacco breeders had made great progress in using anther or microspore to induce haploids in vitro culture which is still in continuous improvement day by day [6]. However, through a large number of studies, it is found that the yield and quality of anther derived haploids tend to deteriorate seriously after doubling, and the value of breeding utilization is limited. In addition, the doubling frequency of such haploids is very low, usually only about 1%. Therefore, so far this method has been rarely used in tobacco breeding [7].
Another way to produce haploid in tobacco is to culture ovary or ovule in vitro. Haploid of egg cell origin is known as female haploid. This haploid has a small variation in ploidy and traits after doubling and the progeny is relatively stable [8]. The technique is suitable for male sterile plants because the ovaries or ovules are cultured as explants and no pollen is involved [5]. Even if seeds are not available from female double haploid plants of male-sterile plants, tissue culture techniques can be used to propagate seedlings, either directly for production or as hybrid parents. They compared the double haploids of female and male haploids of tobacco with their original parents, and found that female double haploids were superior to male double haploids and female double haploids were more similar to their original parents and would not cause inferior yield and quality changes [2]. If the hybrid line is cultured with unfertilized egg cells, the obtained diploid line seems to be similar to the conventional self-crossing line [4], which has been applied successfully in tobacco breeding.
The tobacco domestication scholars Zhu Zhongchun, Wu Haishan and others have carried out several studies in unpollinated ovary culture of common tobacco varieties like Copus yeusuheku No.4, NC2326 and Gexin No.5. The ovaries have been used as explants and cultured on H medium supplemented with auxin IAA 0.5 mg/L and cytokinin KT 2 mg/L. Only Copus yeusuheku No.4 variety could obtain haploid plants from ovary culture. Subsequently, Wu Boji and Zheng Guochang conducted unpollinated ovary culture using improved H medium (supplemented with auxin IAA 0.5 mg/L and cytokinin 6-BA 2 mg/L) for other common tobacco varieties included Liuye, Jinxing, Huangmiaoyu and Gansu Huanghua, and successfully obtained haploid plants. Moreover, used different culturing medium (N6 Medium) supplemented with auxin IAA 0.5 mg/L, cytokinin KT 6 mg/L, inositol 100 mg/L and sucrose 8 g/L) to culture non pollinated ovaries of the large leaf yellow tobacco, and successfully obtained regenerated plants, but the frequency was very low, accounting for only 4%. Although increasing the amount of auxin in the medium could induce vigorous callus and encouraged the differentiation of a large number of seedlings, it produced a variety of chromosome number aberrations, and decreased the frequency of haploid plants. Cultured the non-pollinated ovary of common tobacco variety NC89 in H medium supplemented with 2,4-D and 6-BA, and the results showed that different concentrations of 6-BA had different effects on ovary growth. When the concentration of 6-BA was 8.89 μmol/L, ovary could directly generate embryoid and then form seedlings. Even though all above studies used the ovary as explant focusing on pollen development period as the basis of ovary culture period, each research’s conclusion is different. This may not be suitable for the unfertilized ovule culture of male sterile tobacco with male organ degeneration, however it can provide some reference for the unfertilized ovule culture of male sterile tobacco.
Previous reports have also involved in the culture of unpollinated ovaries of male sterile tobacco [6-8], but it is only limited to pure line varieties. So far, there is no report on the culture of unpollinated ovaries of male sterile hybrids, especially the research in obtaining haploid plants of male sterile tobacco through unfertilized ovule culture. On the basis of previous studies, this research was carried out based on the technology of non-pollinated ovary culture of KRK26, a high quality male sterile flue-cured tobacco hybrid, aiming optimize the culture system by determining the most suitable developmental stage of ovary, and through the selection of different medium types. This study provides a reliable theoretical basis and stable practical technology for haploid plant induction from tobacco ovule at high frequency.
Materials and Methods
Experimental materials
The unpollinated ovules of male sterile flue-cured tobacco hybrid KRK26 introduced from Zimbabwe were used as experimental materials. In order to ensure that ovules can be obtained during the whole experiment, tobacco was sown and potted in two stages, with an interval of 30 days. The inner diameter of the pot was 30 cm, and 100 plants were potted each time.
Experimental methods
Determination of suitable development period and explant form of unfertilized ovule culture
At the flowering stage, five different developmental stages were selected according to different stages of flower buds termed K1-K5 (Figure 1). Ovules were cultured to screen the optimal development stage via in-vitro culture. Ovaries of five developmental stages (K1-K5) were sectioned with a thickness of 6-8 μm and fixed with paraffin according to the previous method. The development of embryos sac was observed and photographed under a microscope (DM 2500, Leica, Germany) at every stage, and the optimal development period was identified at the cell level. The flower buds collected from the above five periods were sterilized in 0.1% mercuric chloride solution for 7-9 min, and washed with sterile water for 3-4 times. Then the base of the flower buds was cut off after disinfection under sterile conditions and the ovary was extruded with tweezers. The ovaries of each stage were divided into three parts for different treatments which are circumcision, removal of ovary wall and direct ovule stripping. The treated ovaries were placed on different culture media.
Figure 1: Five different developmental stages of flower buds used for ovule culture
K1: The flower buds are about to open; K2: The corolla is three times longer than the calyx; K3: The corolla is two times longer than the calyx; K4: The corolla is less than 1 cm longer than the calyx; K5: The corolla is about to reveal the calyx.
Selection of suitable medium for unfertilized ovule culture
Based on H medium reported by [5], H medium (represented by HW) and N6 Medium [7] improved by five optimized medium formulations were set up for ovule culture in vitro. Using the circumscribed ovaries, ovaries with wall removed and ovules directly stripped as explants, embryoid induction was carried out to select the optimal medium for subsequent haploid induction. The optimized formulas are shown in Table 1.
Induction of regenerated haploid plants
The optimal medium and the most suitable explant were selected for embryoid induction. According to the actual situation of embryoid induction accompanied with previous research experience, the medium formula was fine-tuned to facilitate the induction of regenerated buds. The fine-tuning of medium formula included five treatments: (1) three kinds of formula were designed including hormone free, half inorganic salt content and hormone free with half inorganic salt content. The medium without hormone removal and half inorganic salt content was used as control to compare the embryoid development; (2) The sucrose content in the medium was increased from 2% to 4% to observe the effect of different sucrose contents on the embryoid development; (3) The agar concentration in the medium was increased from 0.8% to 1.0% to observe the effects of different agar concentrations on embryoid development; (4) The content of cytokinin in the medium was reduced from 2 mg/L to 1.5 mg/L to observe the effect of the change of cytokinin content on embryoid development; (5) The pH of the medium was increased from 5.5 to 5.8 to observe the effect of pH change on embryoid development. According to the experimental results, the final formulation of embryoid induction medium and regenerated bud induction medium were determined. In order to induce the embryoid to produce regenerated bud, the embryoid was transferred to the final medium for bud induction. Finally, regenerated buds were transferred to H medium or N6 medium supplemented with 10 mg/L IAA to obtain regenerated plants. Embryoid induction, bud regeneration induction and rooting induction were all placed in a 28°C light culture chamber less than 1500 lux light for 10 h every day.
Identification of regenerated haploid plants
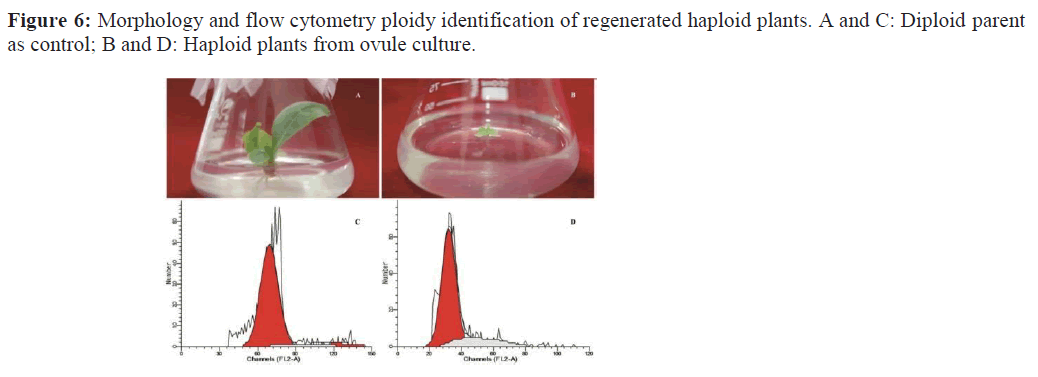
When the regenerated plants grew to 2~3 cm in height or had 3~4 leaves, the leaves of the regenerated plants were collected and the ploidy was determined by BD FACScalibur flow cytometry produced by BD Company in the United States. Specific methods are as following: young leaves of about 1.3~1.6 cm2 of regenerated plants were taken and shredded with a blade in 0.5 mL of cell lysate (Tris-HCl buffer, pH =7.2). The sample with the solution mixture was filtered into a test tube through a microporous membrane with a diameter of 30μm, and stained with 20 uL DAPI staining solution (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, 0.4 μg Propidium Iodide (PI) (Sigma), 0.1% TritonX-100 and 4 μg RNase A) for 15 min, then the sample was applied to the flow cytometer. The normal ovary DNA donor plant content was used as a control standard to adjust to 40 channels. At least 10000 cells were analyzed for each curve peak and repeated 4 times.
Results
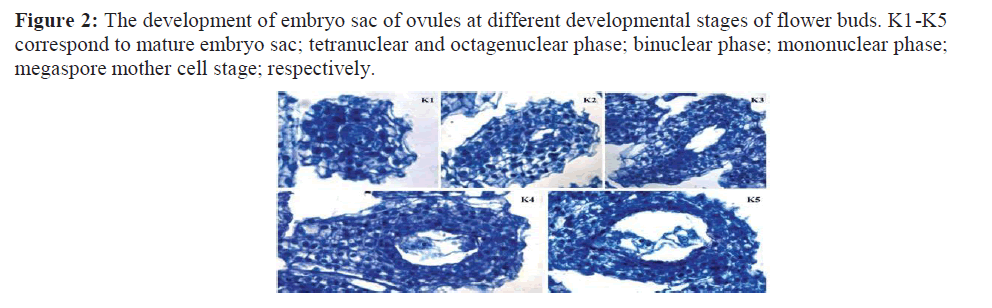
Determination of suitable development stage of the unfertilized ovule culture
According to the observation of paraffin sections (Figure.2), five different stages of the flowering buds (K1-K5) were basically characterized according to the ovule’s development stage as follows: K1-mature embryo sac; K2-tetranuclear and octagenuclear phase; K3-binuclear phase; K4-mononuclear phase; K5-megaspore mother cell stage.
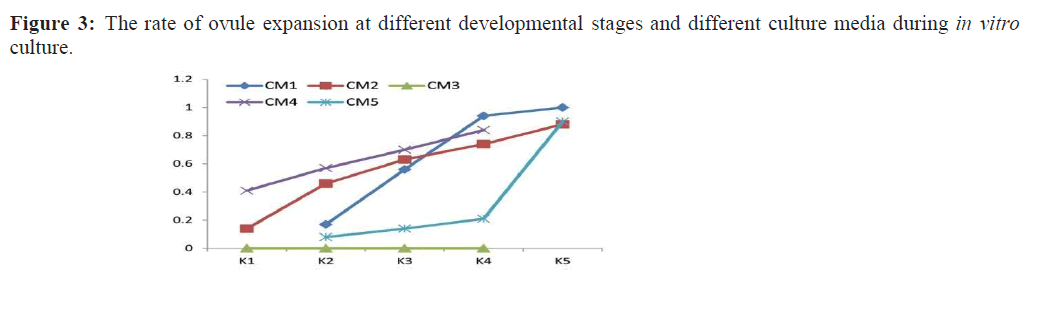
After ovaries collection and sterilization, ovaries used as explants were treated in different ways including circumcision of ovaries, removal of ovary wall and direct exfoliation of ovules. Circumscribed ovaries were obviously expanded after 7 days of inoculation and culture, ovaries with removed wall became green and thickened in which the rupture of ovary wall was not observed, while the inner ovules showed no sign of expansion. At this time, the ovary wall was removed, and the ovules still browned and died in the process of culture. After removing the ovary wall and stripping the ovule directly, the ovule was significantly enlarged after inoculation for 7 days. The proportion of explants expansion during ovule culture at different developmental stages is shown in Figure. 3.
It can be seen from Figure.3 that the ovule expansion rate of each development stage from K1 to K5 in different media showed an upward trend. The ovule at K5 stage showed the best performance in CM1, CM2, and CM5 media, almost all the K5 ovules expanded, and the proportion of the expanded ovules was the highest among the different developmental stages. Therefore, the more mature the embryo sac was, the worse the ovule proliferation induction was. In general, the best stage for ovule inoculation range between K3 to K5 which are from the megaspore mother cell to the binuclear stage. Therefore, it is better to pick the bud and collect the ovules in early developmental stage.
Optimization of suitable medium for unfertilized ovule culture
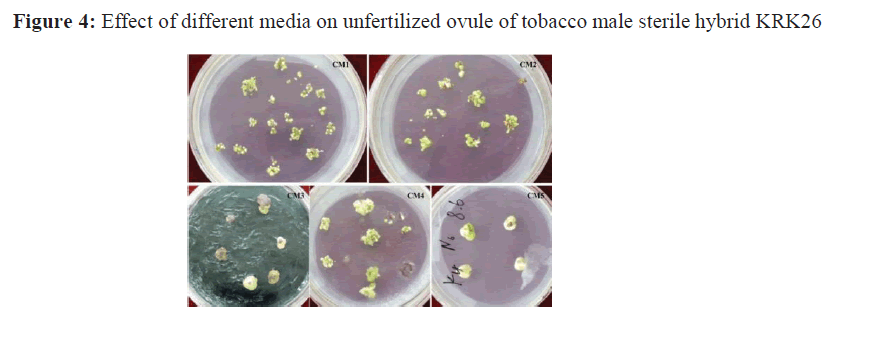
The five media listed in Table 1 were used to culture unfertilized ovules. Results showed that the CM1, CM2 and CM4 media showed better effects on ovule culture, but the culture effect of CM1 was the best (Figure.3 and Figure.4). When the basic medium was HW, the induction effect was slightly better in the medium supplemented with cytokinin 6-BA (CM4) than that of the medium supplemented with KT (CM2) (Figure. 3). When ovules cultured on CM1 and CM2 media, a large number of embryoids produced by direct embryogenesis, while ovules cultured on CM4 (supplemented with IAA 0.5 mg/L + 6-BA 2 mg/L) could not directly produce embryoids, in which formed calli first then differentiated into embryoids. Although, ovules in CM4 medium could ultimately develop seedlings in indirect way, the induction time of embryoids was prolonged which is not preferred. On N6 Medium, except the ovules of K5 stage, the rate of ovule expansion was very small and the browning death of explants was serious. However, the effect of CM3 medium with activated carbon was the worst. When ovaries were used as explants, the ovaries wall became green and thick and no obvious expanded ovules were detected whether in ovaries or ovules.
| Medium number | Formula |
|---|---|
| CM1 | H+IAA 0.5 mg/L+KT 2 mg/L+agar0.8%+sucrose2% |
| CM2 | HW+IAA 0.5 mg/L+KT 2 mg/L+agar0.8%+sucrose2% |
| CM3 | H+ Activated carbon C 10 g/L+ IAA 0.5 mg/L+agar0.8%+sucrose2% |
| CM4 | HW+IAA 0.5 mg/L+6-BA 2 mg/L+agar0.8%+sucrose2% |
| CM5 | N6+ IAA 0.5 mg/L+KT 6 mg/L+inositol100 mg/L+agar1%+sucrose8% |
Table 1: Optimization design of culture medium for unfertilized ovule culture
Induction of regenerated haploid plants
Based on the optimized conditions, the ovules of KRK26 non pollinated flower buds were collected at three developmental stages, K3-K5, directly stripped and inoculated on CM1 medium. After 15 days, the ovules were obviously expanded (Figure.5, A) and embryoids were obtained after 30 days of culture. The obtained embryoids were cultured for about 14 days, and the embryoids began to elongate obviously forming two poles (Figure.5,B). Subsequently, most of the polar embryos differentiated into cotyledon like embryos, and a few of them were similar to seedlings (Figure.5, C; D). The generated seedlings were placed on rooting medium to promote root growth (Figure.5,E). After 14 days, the regenerated seedlings began to form roots and grow well, and the complete regenerated plants could be seen after 20 days (Figure.5, F; G).
It was found that some of the embryoids were vitrified during embryoid induction. The author tried to increase sucrose content from 2% to 4%, pH from 5.5 to 5.8 and agar concentration from 0.8% to 1.0% and reduce cytokinin content from 2 mg/L to 1.5 mg/L to inhibit the occurrence of vitreous shoots. The results showed that all of these methods could inhibit the occurrence of vitreous shoots, but all of the other media were unfavorable to the growth of embryoid except 1% agar. Therefore, the concentration of agar in CM1 medium was adjusted to 1% for later embryoids induction and buds regeneration.
It was also found that although CM1 medium could induce a large number of embryoids through direct generation, the embryoids gradually browned during the culture process. Therefore, we tried to transfer the embryoids to 1/2 CM1 without hormones (i.e. the inorganic salt content of CM1 medium was reduced by half) and CM1 without hormone (i.e. H medium) for further culture. The results showed that by the removal of hormones from the CM1 medium, embryoids didn’t turn brown. At the same time, the embryoids could elongate and form whisker leaves, and develop slowly. On the hormone free medium CM1, some embryoids induced by ovules at K3, K4 and K5 stages gradually developed into thin seedlings (i.e. regenerated buds). The regenerated buds then were transferred to H or N6 medium supplemented with IAA 10 mg/L and it was found that the regenerated buds could grow well and form roots quickly, and obtain a large number of regenerated plants.
Haploid identification and plant performance
A large number of plants were obtained by ovule culture (Figure.7, A). The ploidy of regenerated plants could not be determined because of the different morphology of the obtained plants, so the ploidy of regenerated plants was identified by flow cytometry (Figure.6). Plants obtained in this study included haploid plants; some of them grew slowly and form fewer leaves and shorter plant height far less than those of their parents. Some haploid plants showed terminal bud necrosis after long culture time (Figure.7, B). The haploid plants transferred to the greenhouse for cultivation were male sterile and could bloom normally, however their growth was weak (Figure.7, C and D).
Discussion
Our results showed that the unpollinated ovules of tobacco could successfully develop into mature plants under optimized conditions. We also could develop haploid plants directly without callus formation, which could avoid chromosome number aberrations caused by callus regeneration. These results are consistent with the results reported by in which haploid plants were induced by non-pollinated ovaries, however the wall of the non-pollinated ovaries became green and thick with no sign of expansion in the inner ovules. To avoid this phenomenon, therefore, we removed the wall of ovaries before the inoculation, however the ovules turned brown and died in the process of culture. We hypothesized that, if we removed the wall of ovaries and inoculated them again or stripped the ovules directly, we could observe the ovule expansion, and it differentiated into many polar embryos and gradually grew into plants. This may be due to the use of male sterile materials.
Collected unpollinated ovaries for culture at the late uninucleate or binucleate stage of pollen, which could achieve good induction effect and obtain regenerated plants [6,7]. However, the authors used male sterile material, which is not suitable to determine the exact stage of the collected explants according to the development period of pollen. Therefore, the development period of ovule was judged by the appearance of flower bud. The results showed that the more mature the embryo sac was, the worse the ability of ovule proliferation induction was. The ovule developed earlier from megasporocyte stage to binucleate stage, from the time when the corolla of the flower is about to expose the calyx to the time when the Corolla is twice longer than the calyx, showed the best ability of ovule induction. Accordingly, we propose three possible origins of generated embryoids resulted from ovule culture at these stages. Firstly, the embryoids might be directly originated from megasporocytes, which undergo meiosis, proliferate to form cell clusters and then develop into embryoids. Secondly, the embryoids could be derived from megaspore cells that divide and proliferate to form cell clusters and then develop into embryoids. Finally, the generated embryoids might be originated from egg cells. After ovule inoculation, normal mature embryo sac will be formed following the development path of embryo sac, and then the egg cells in it will develop into embryoids [8].
Following [6] who used N6 medium to obtain good culture results, we used N6 medium (CM5) for ovules culture of different developmental stages, but the ratio of the expanded ovules was very low in all stages except the ovules at K5 stage, browning death phenomenon of explants was serious and no regenerated plants were obtained. The reason may be explained as following: (1) The nutrient composition of basic culture N6 is different from that of H medium; (2) The concentration of agar was 20% higher than that of other types of media, made it hard which restricts nutrients absorption; (3) The concentration of sucrose was 8% which was three times higher than other types of media. In addition to providing carbon source, another important role of sucrose in culture is to regulate osmotic pressure and affect the absorption of substances by cells also confirmed that sucrose concentration higher than 4% inhibited the induction of embryoids. In the unpollinated ovary culture of cucumber, the low sucrose concentration was beneficial to the division and proliferation of cells in embryo sac, thus inhibiting ovule expansion.
Among the different media used in this study, CM1 medium was the best, followed by CM2, in which haploid plants were obtained directly. This is consistent with the results reported by [6,7]. The ovules development in CM4 medium (IAA 0.5mg/l + 6-BA 2mg / L) was also better, that could sprout through indirect way, which was consistent with the result reported by [8]. In brief, the culture of unpollinated ovules of tobacco can be divided into direct and indirect ways. The direct way is that the ovules develop directly into embryoids and then into seedlings, while the indirect way is to form callus first, then differentiate into embryoids and finally into seedlings. Among all kinds of the tested culture media, CM3 with activated carbon had the worst culture effect, and the reasons need to be further explored.
Conclusion
An optimized culture system was established to induce haploid plants from unfertilized ovules of tobacco male-sterile hybrid (KRK26). Ovules from megasporocyte stage to binucleate stage, the most suitable stages for ovule culture, were collected as explants and inoculated on to H medium supplemented with auxin IAA 0.5 mg/L and cytokinin KT 2 mg/L, the most appropriate medium for embryoids induction. The final concentration of sucrose and agar was 2% and 1%; respectively, the most adequate concentrations. Then, the embryoids were transferred to H medium without hormones for further culture to induce regenerated buds. The final concentration of sucrose and agar in the regenerated bud induction medium was also 2% and 1%, respectively. Subsequently, the regenerated buds were cultured on H medium or N6 medium supplemented with auxin IAA 10 mg/L to promote rooting and to obtain regenerated plants. The final concentration of sucrose and agar in the rooting medium of regenerated buds was 8% and 0.8%, respectively. Finally, the ploidy of regenerated plants was identified and haploid plants were selected for further studies.
References
- Jacquier NMA, Gilles LM, Pyott DE, Martinant JP, Rogowskyet PM, et al. (2020) Puzzling out plant reproduction by haploid induction for innovations in plant breeding. Nat Plants 6: 610-619.
[Crossref], [Google Scholar]
- Wang L, Qiao J, Shi Y, Li S, Miao B (2015) Research progress on isolated microspore culture of Eggplant. Northern Horticulture 13: 190-194.
[Crossref], [Google Scholar]
- Wang Y, Cheng L, Liang Y, Lu X, Zhang (2017) Isolation and culture of pollen tetrad protoplasts from Solanum tuberosum. American Journal of Potato Research 94: 417-424.
[Crossref], [Google Scholar]
- Puzzling out plant reproduction by haploid induction for innovations in plant breeding.
- Wernsman EA, Matzinger DF, Rufty RC (1989) Androgenetic vs. gynogenetic doubled haploids of tobacco. Crop Sci 29: 1151-1155.
[Crossref], [Google Scholar]
- Zhu, Z, Liu Z, Wu H, An Q (1981) Embryoid development of unpollinated tobacco ovary in vitro. Acta Botanica Sinica 23: 499-501.
[Crossref], [Google Scholar]
- Wu, B, Zheng G (1982) Cytological and embryological studies on haploid plants induced from unpollinated tobacco ovaries. J Integr Plant Biol 24: 125-129.
[Crossref], [Google Scholar]
- Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163: 85-87.
[Crossref], [Google Scholar]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences