An Innovative Approach to Solar Thermal Water Pasteurisation
Ilan Adler*, Hippolyte Gavilan, Peter McKay and Luiza C Campos
Department of Civil, Environmental and Geomatic Engineering, University College London, Gower Street, London, UK
- *Corresponding Author:
- Ilan Adler
Department of Civil, Environmental and Geomatic Engineering
University College London, Gower Street, London, UK
Tel: 02076790536
E-mail: ilan.adler.09@ucl.ac.uk
Received Date: January 01, 2018; Accepted Date: January 22, 2018; Published Date: January 25, 2018
Citation: Adler I, Gavilan H, McKay P, Campos LC (2018) An Innovative Approach to Solar Thermal Water Pasteurisation. J Environ Res Vol.2: No. 1: 1.
Abstract
Despite safe access to drinking water being recognized by the UN as a basic human right, millions of people around the globe lack adequate supplies, with water-related diseases still being a major cause for mortality, particularly in developing countries. The aim of this study is to test a novel, low-cost solar pasteurisation technology, which has a built-in function to self-regulate according to available sunshine, in order to assess its suitability for safely processing water for drinking purposes. The device, known as a passive solar thermal pasteuriser, can be applied in environments where it is critical to pasteurise the water by reaching sufficient temperatures. A prototype was tested under laboratory conditions, with lamps calibrated to provide representative solar irradiances. Experiments under varying conditions show temperatures as high as 76°C were reached, which according to the literature is sufficient to pasteurise water in less than 5 minutes, providing safe drinking water and eliminating common waterborne pathogens. Future tests and field trials are currently under way to assess microbiological efficiency and improve performance.
Keywords
Water purification; Solar energy; Pasteurisation; Disinfection; Sustainable development
Introduction
In 2010, the United Nations (UN) General Assembly and the UN Human Rights Council recognised access to safe drinking water as a human right. Nevertheless, 748 million people worldwide were still without access to improved water sources in 2014 [1]. Additionally, the World Health Organization (WHO) and UNICEF reported that water-related diseases are a major cause of illness and death around the world [2]. It is therefore safe to conclude that effective low-cost disinfection devices have much room for improvement and a consequent opportunity for development in emerging economies.
Chlorination of water is the most common form of water treatment worldwide [3] but is widely associated with harmful disinfection by-products and continuous operational costs [4]. Slow sand filters and household filters are equally appropriate for drinking purposes, but require constant maintenance costs and frequent replacement of filters [5]. UV water disinfection is equally a good option but requires maintenance and most importantly power supply.
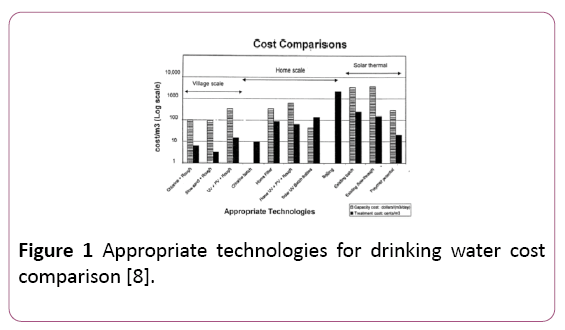
Thermal disinfection was named “pasteurisation” after Louis Pasteur (1822-1895). Pasteurisation by boiling water is widely recognised as a safe way to treat water from major pathogens dangerous to human health [5]. However, boiling water requires fuel, which is an unsustainable and often costly option. Despite this, it was proven that pasteurisation can take place at temperatures under 100°C. Solar pasteurisation has been safely achieved with values as low as 65°C for a period of 6 minutes [6]. However, the main drawback of current thermal pasteurisation methods is cost [7], as shown in Figure 1.
The aim of this study is to test the capacity of a novel technique for solar pasteurisation in order to assess its suitability for safely processing water for drinking purposes in developing countries. The device is to be used in environments where it is critical to pasteurise the water by reaching sufficient temperatures. Depending on what temperatures are reached and for how long, this would then provide safe drinking water [6]. In addition, conclusive results will allow for the pasteurisation device to be combined with a Passive Solar- Thermal Pumping system, currently being developed at University College London (UCL). This low-cost technology can be used for purifying drinking water, cooking or alternatively as a fuel-saving device, used to heat up water as an alternative to mainstream expensive, unreliable and unsustainable options.
Methodology
Passive solar thermal pasteuriser
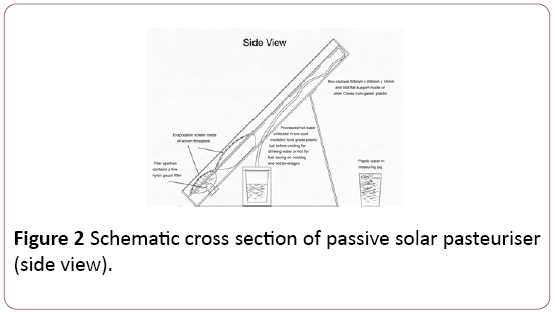
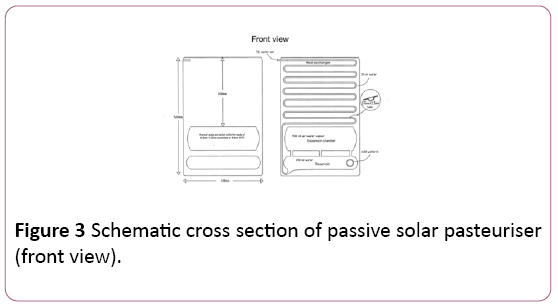
The novel feature of The Passive Solar Thermal Pasteuriser is an internal pumping mechanism which only pumps water through the heat-exchanger panel when there is enough solar irradiance to heat the water to a pasteurising temperature, acting in a self-regulating manner.
The water in the reservoir at the bottom of the unit is preheated by solar radiation before being pump up through the heat-exchanger by vapour pressure from water on the evaporation screen in the expansion chamber, after passing through the heat-exchanger the water exits the device as pasteurised hot water.
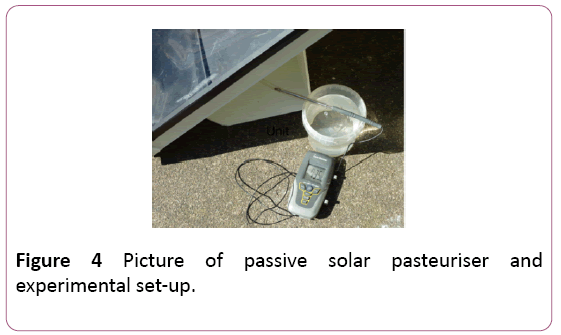
It is then collected in an insulated food grade plastic tub. A schematic of the device is represented in Figures 2 and 3, with an outdoor testing arrangement shown in Figure 4.
Monitoring performance parameters
The different experiments were run at UCL’s environmental engineering laboratory, under consistent environment conditions and ambient temperatures of 21°C. For each experiment, the levels of irradiance were measured at the highest point where the device receives light, in the middle (25 cm down) and at the lowest point. All three positions were at the middle of the device on the horizontal axis.
Water started flowing out of the pasteuriser at similar times after the start of each experiment: after 8 minutes in experiment 1, 2 and 4, and after 70 minutes in experiment 3 (or 10 minutes after putting the pasteuriser back to its working position).
Experiment 1
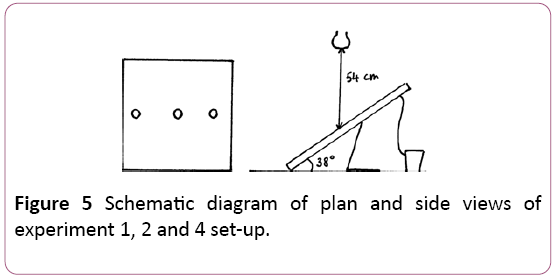
The passive solar pasteuriser was set up in a consistent manner throughout experiments 1, 2 and 4. As described in Figure 5, it was installed at an angle of 38° from the support plane.
The device was placed between a back support and a front metal piece preventing it from sliding and providing a point of reference for the device to be placed it in the same position for each experiment.
A set of three lamps was constructed and set up at a vertical distance of 54 cm above the pasteuriser, as represented in Figures 5 and 6. These three lamps were set in line, at a distance of 20 cm from each other.
The central lamp was a Philips 240 Volts, 500 Watts, whereas the two lamps at each extremity of the set were Philips heat lamps of 250 Volts, 250 Watts. These lamps were installed to imitate the sun’s luminosity and heat, representing outdoor conditions in which the pasteuriser would be used.
Aluminium foil paper was installed on the side facing the device, from the lamps down to the bottom of the pasteuriser in order to maximise luminosity and irradiance on the unit (Figure 7).
In order to understand the temperatures reached inside the pasteurising unit, a thermometer was placed inside the top of the device. In this report, the measurements for this thermometer are referred to as the temperature in the pasteuriser.
For each experiment, the volume of water placed in the passive solar thermal pasteurising unit was constant at 600 mL. After placing the water inside the device, the experiment started as soon as the lights were turned on.
The results of experiment 1, described in Section 3.1, were collected in two different ways. First, by reading the temperatures given by the thermometer in the pasteuriser. Second, by noting the temperatures of the processed water taken by a thermometer placed inside the tub.
Experiment 2
The only change to notice from Experiment 1 is the increase in irradiance, obtained by applying an extra aluminium foil paper “wall” on one side of the pasteuriser, as shown in Figure 8. This was done in order to focus more light on the device and simulate more optimal weather conditions by increasing the irradiance on the pasteuriser.
The temperature of the pasteurised water was measured using a thermometer at 10 minute intervals, in order to reduce evaporation and temperature drop due to the tub not being fully closed.
Experiment 3
After obtaining results from experiments 1 and 2, it was decided to test the solar pasteuriser in a flatter position. The optimal angle and direction for the pasteuriser to work would be facing the sun. Therefore this set-up would simulate better solar conditions and higher, more even irradiance across the unit. In addition to changing the angle of the panel to 20°, the unit was raised up closer to the lamps, at a direct vertical distance of 36 cm.
A third “wall” made of aluminium paper was also put in place in order to focus all the available light from the lamps on the pasteuriser, therefore closing up the entire set-up.
After testing the pasteuriser in this position for 60 minutes, it was placed back in its original position (Section 2.1) due to inconclusive results described in Section 3.2. The experiment therefore continued for 30 minutes more, at the same position it was in for experiments 1, 2 and 4. In this experiment, the pasteurised water temperature was measured with a thermometer directly while exiting the unit, allowing for a more accurate estimate of the water temperature reached.
Experiment 4
In experiment 4, the pasteuriser was set up in the same manner as in experiments 1 and 2. The 3 sides of aluminium foil paper all around the device optimised irradiance were maintained. Furthermore, the water temperature was measured directly when exiting the pasteuriser, as was done in experiment 3.
Results
Irradiation levels for experiments 1, 2, 3 and 4 are detailed in Table 1.
| Irradiance (W/m2) | ||||
|---|---|---|---|---|
| Pasteuriser zone | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 |
| Top | 820 | 900 | 930 | 1060 |
| Middle | 960 | 1030 | 1020 | 1080 |
| Bottom | 560 | 580 | 680 | 680 |
Table 1: Experiments 1 and 2 irradiance levels on pasteuriser surface.
Experiments 1 and 2
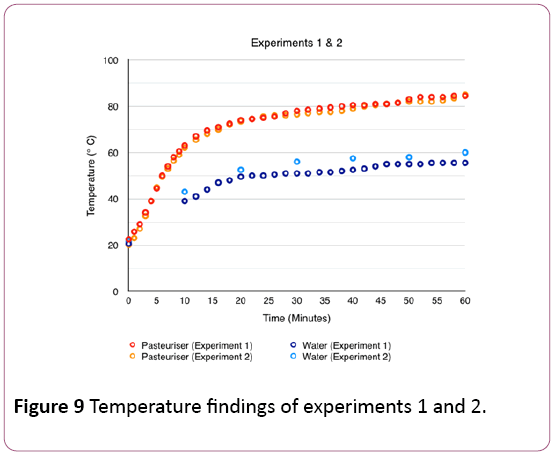
In experiments 1 and 2, the results show the temperatures in the pasteurising unit, as well as water temperature in the tub collecting the processed water. Figure 9 shows the variation of temperatures inside the pasteuriser as well as in the insulated food grade plastic tub for both experiments.
Although the irradiance levels were relatively different in experiments 1 and 2, the temperatures in the pasteuriser increased similarly with time, starting at 22.5 and 20°C and ending at 84.5 and 85°C respectively.
The temperatures in the water collector, though, varied more. 10 minutes after the start of the tests, the water temperatures were 39°C and 43°C for experiments 1 and 2 respectively, rising up to 55.5°C and 60°C. This difference can be explained by higher levels of irradiation in experiment 2 than in experiment 1, as well as taking measurements at 10- minute intervals in experiment 2, preventing the water from cooling as quickly.
Experiment 3
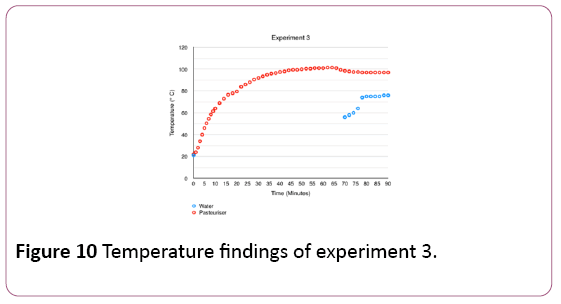
As explained in Section 2.2, irradiation levels for the first 60 minutes of the experiment are higher and more uniform than in the previous two experiments due to the extra wall placed around the device and its angle. In the last 30 minutes of the experiment, the irradiance levels are the same as for experiment 4, detailed in Table 1.
The graph in Figure 10 shows very high temperatures inside the pasteuriser, peaking at 101.5°C between minute 62 and 64 of the experiment. Furthermore, the graph shows water temperatures reaching up to 76°C between minutes 88 and 90 of the test. However, the pasteuriser did not work at this flatter angle. Consequently, the unit was placed back to its original position at minute 60, at an angle of 38° and at a distance of 54 cm to the lamps. The difference in water temperatures obtained in experiment 3 compared to experiments 1 and 2 can be explained by higher irradiance levels and by the fact that temperatures were measured directly when water exited the pasteuriser.
Experiment 4
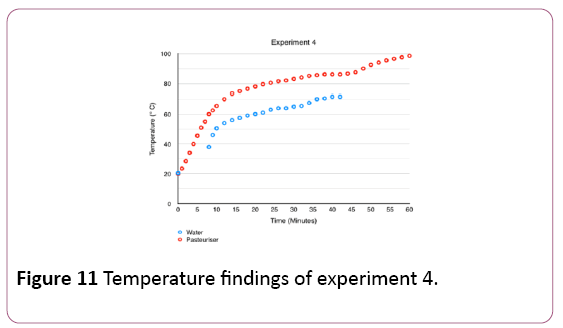
Figure 11 shows temperatures inside the pasteuriser peaking at 98.5°C after 60 minutes. Furthermore, the graph shows water temperatures reaching up to 71.5°C between minute 40 and 42, when the pasteuriser stopped releasing water.
Discussion
According to Feachem et al. [8] the highest temperatures obtained in experiments 1 and 2 (up to 60°C) would require staying at those levels for more than 1 hour. However, the values obtained are not representative of the actual highest temperature reached by the water. This is because the measurements were taken from the food grade plastic tub, rather than directly exiting the pasteuriser, which caused the water to cool down quickly after processing.
Additionally, the water temperatures reached in experiments 3 and 4 (76 and 71.5°C respectively), measured directly at exit of the pasteuriser would be sufficient to provide safe drinking water with a relatively short retention time at these temperatures, as detailed by Feachem et al. [8] and Andreatta [7]. Both report that water reaching 72°C should be maintained at these temperatures for only 6 minutes to insure safe drinking water.
Furthermore, it is important to note that the experiments were run with water and air at around 20°C (Table 2) compared to what could be experienced in developing countries. Higher initial air and water temperatures would allow for higher output temperatures to be reached faster.
| Initial Temperatures (°C) | ||
|---|---|---|
| Experiment | Water | Pasteuriser |
| 1 | 21 | 22.5 |
| 2 | 20 | 20 |
| 3 | 21 | 22 |
| 4 | 20.5 | 20.5 |
Table 2: Experiments 1 and 2 irradiance levels on pasteuriser surface.
Finally, the differences in the highest temperatures reached in experiments 3 and 4 (4.5°C difference) suggest that it may be a more suitable solution to start pasteurising the water when the device is already at a high temperature, rather than filling the pasteuriser at room temperature with little to no exposition to sunlight, before installing it outdoors for processing. Therefore, ideal conditions might comprise positioning the device outdoors before the start of pasteurisation, allowing it to heat up first.
Conclusion
In conclusion, the first experiments done on this passive solar thermal pasteuriser are promising, as temperatures as high as 76°C have been reached. These temperatures are believed to pasteurise water in less than 5 minutes, providing safe drinking water, and eliminating all common waterborne harmful pathogens, bacteria and viruses [9].
However, it is necessary for further tests to be done in order to make affirmative conclusions on the potential of this technology. Different lamps, dispositions, angle and distances should be tested in order to replicate diverse climate conditions. A wider range of air and water temperatures at the beginning of each experiment should be tested in order to understand their implications on the device’s performances. It is also important to compare solar conditions obtained in the experiment to the ones in countries where the device could be used in order to insure adequate irradiance levels.
It is crucial to simultaneously measure water temperatures directly while exiting the device as well as in the recipient tub in order to truly understand how long certain batches of water remain at adequate temperatures. In general, more experiments should be undertaken in order to standardize the performance levels of the pasteuriser by applying standard deviation and linear regression data analysis for similar tests. Equally, it is important to carefully measure volume coming out of the pasteuriser at regular intervals, as flow rate is an important factor regarding the device’s performance. The volumes measured in this study are not relevant as they were taken on irregular intervals and were influenced by evaporation.
Finally and most importantly, it is notable that for all four experiments, the maximum irradiance is observed in the middle of the pasteuriser, as it comprises the area closest to the lamps. It is crucial to note that indoor artificial lighting cannot provide lighting comparable to sunlight. The device should therefore be tested outdoors, ideally in a country with similar climate to where it will to be used, in order to get a true sense of its capacity to produce safe drinking water. It is also important to note that the device does not function at a low angle or in a flat position and different angles should be tested in order to determine the best possible position.
Acknowledgments
This work has been partly funded by the UCL Grand Challenges for Global Health, obtained by Drs Ilan Adler and Luiza Campos in 2016 and by a subsequent grant from Lloyds Insurance (UK), 2017.
The authors also greatly acknowledge the support of the lab technicians at the UCL Environmental Engineering Laboratory, namely: Dr. Judith Zhou, Dr. Melissa Canales, Mr. Ian Seaton and Mr. Dave Kruup, for their invaluable support to the project.
Author Contributions
Dr. Adler supervised the project and led the research team, as well as edited the final version of the paper.
Mr. Gavilan conducted most of the experiments in the lab, as an MSc student under Dr Adler’s supervision (2016).
Mr. McKay provided valuable input for designs as well as schematic diagrams.
Dr. Campos proofread the paper and assisted in supervising and setting up the studies.
Conflicts of Interest
The authors declare no conflict of interest. Furthermore, the UCL Grand Challenges of Global Health (the founding sponsors) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results. A patent registration for the technology is currently in progress.
References
- WHO, UN-Water (2014) Global analysis and assessment of sanitation and drinking-water GLAAS 2014 Report: Investigating in water and sanitation: Increasing access, reducing inequalities. World Health Organization. Geneva, Switzerland.
- WHO/UNICEF Joint Monitoring Programme (2000) Global water supply and sanitation assessment 2000 Report. World Health Organization and United Nations Children's Fund. USA.
- Niewoehner J, Larson R, Azrag E, Hailu T, Horner J, et al. (1997) Opportunities for renewable energy technologies in water supply in developing country villages. National Renewable Energy Laboratory, Golden, CO.
- Xie Y (2004) Disinfection byproducts in drinking water: Formation, analysis, and control. CRC Press, USA.
- Burch JD, Thomas KE (1998) Water disinfection for developing countries and potential for solar thermal pasteurization. Solar Energy, 64:87-97.
- Rochester Institute of Technology (2007) EPA water disinfection project. Rochester Institute of Technology. Rochester, New York, USA.
- Andreatta D (2007) A Summary of Water Pasteurization Techniques. Columbus, OH.
- Feachem RE, Bradley DJ, Garelick H, Mara D (1983) Sanitation and disease: Health aspects of excreta and wastewater management. Wiley, New York, USA.
- https://www.sswm.info/content/solar-pasteurisation
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences