Active Bio Moieties and Pharmacological Properties: A review on Acacia ataxacantha
Krupanidhi AM*, Prakash Dabadi, Mujeebulla RH, Naipunya B, Lipishree BM, Sharath GR and Shreyas RA
Department of Pharmacology, Rajiv Gandhi University of Health Sciences, Karnataka, India
- *Corresponding Author:
- Krupanidhi AM
Department of Pharmacology,
Rajiv Gandhi University of Health Sciences,
Karnataka,
India,
Tel: 9845825488;
E-mail: dramkrupanidhi@gmail.com
Received date: October 03, 2022, Manuscript No. IPPRR-22-14656; Editor assigned date: October 06, 2022, PreQC No. IPPRR-22-14656 (PQ); Reviewed date: October 21, 2022, QC No. IPPRR-22-14656; Revised date: December 28, 2022, Manuscript No. IPPRR-22-14656 (R); Published date: January 05, 2023, DOI: 10.36648/IPPRR.6.1.001
Citation: Krupanidhi AM, Dabadi P, Mujeebulla RH, Naipunya B, Lipishree BM, et al. (2023) Active Bio Moieties and Pharmacological Properties: A Review on Acacia Ataxacantha. J Pharmacol Rev Rep Vol:6 No:1
Abstract
In present scenario the reviewing of various medicinal plants are necessary to gain the knowledge of importance of phytoconstituents and its therapeutical value. Based on this concept we focused on review of various biological and pharmacological activities of whole part of the Acacia ataxacantha. This plant is widely distributed throughout the tropical and subtropical region of Karnataka state. The Acacia ataxacantha plant of its bio compounds were exhibiting various clinical importance. The plant contain various secondary metabolites like mucilage, tannins, saponins, asparagine, alkaloids, flavonoids, sterols, triterpenoids, cardiac glycosides, sesquiterpene’s and essential oil. The plant of Acacia ataxacantha exhibits various pharmacological activities like, based on these evidences the present review was focused to collect and summarizes the overall the brief information’s on various parts of the Acacia ataxacantha and systematically documented the medicinal significances of the plant.
Keywords
Acacia ataxacantha; Flavonoids; Inhibitory effect; Ulcer; GI motility
Introduction
The numerous plant sections employed historically for their known biological qualities and their abundance of nutrient rich food components due to its chemical content, the plant is used medically to treat both acute and chronic ailments. It also contains a variety of antioxidants and phenolic compounds. (Mucilage, tannins, gallic acid, asparagine, alkaloids, flavonoids, sterols, triterpenoids, cardiac glycosides, sesquiterpenes and essential oil) Both the aerial and subsurface components are advantageous [1].
The process of developing new drugs has continued to be significantly influenced by natural products. Natural product characteristics have continued to have a substantial impact on the process of creating new medications. The presence of phytoconstituents i n m edicinal plants contributes to their therapeutic effectiveness in treating ailments.
The more than 1200 species of the cosmopolitan genus Acacia are found all over the world, but tropical and subtropical areas have the highest density and largest diversity.
This species usage in traditional medicine to address of tooth decay, diarrhoea, bronchitis, coughing and joint pain has been documented in Benin, Nigeria and Kenya. There have been reports of using several Acacia species, flowers, leaves, barks and gum to treat various illnesses [2]. Apparently, secondary metabolites with antioxidant, antibacterial, anticancer and antifungal properties include phenolics and flavonoids.
Synonyms: Acacia ataxacantha DC, Acacia eriadenia, Acacia lugardiae.
Literature Review
Historical aspects of Acacia ataxacantha
This plant was previously called Acacia ataxacantha. The specific name Ataxacantha derived from Greek word “taxis” which means arrangement and “acantha” which means thorns.
Geographical distribution
It is common to find Acacia ataxacantha (Fabaceae) over much of sub-Saharan Africa, Nigeria, Kenya and some regions of India like Rajasthan, Gujarat, Maharashtra, Tamil Nadu, Karnataka (some regiosns of Karnataka) [3].
Identi ication of Acacia ataxacantha
A non-climbing shrub or, rarely, a tree up to 10 metres high, with a crown that is frequently somewhat curved in arborescent forms and a trunk that is up to 0.55 metres in diameter. Young branchlets are pale yellowish or grey to reddish-brown, sparsely to densely pubescent and frequently have an indumentum that is slightly golden. Bark is light to dark yellowish or grey-brown, rough, barely fissured and occasionally flaking. Stipules are not spinescent, arranged in groups above the nodes and are quickly become deciduous. Prickles that are dispersed along the internodes and are typically highly recurved, red-brown to purplish and frequently broad based. Flowers are yellowishwhite, pedicellate or seem sessile with spike. Usually, 1/3 to 1/2 as long as the corolla, the calyx is cup-shaped, pubescent to sparingly pubescent and has lobes [4].
Much of sub-Saharan Africa is home to the wide spread Acacia ataxacantha (Fabaceae) plant. This type is a tall, 5 m to 8 m, extremely spiky bush. The leaves are alternating and pinnate, with 5 to 12 sets of pinnae on the spine. Spines on twigs are short and obviously point downward. The white blooms with a lengthy transition are axillary, measuring 3 cm-5 cm long and are placed on a stem measuring 10 mm to 15 mm.
Scientific classification:
• Kingdom: Plantae
• Clade: Tracheophytes, Eudicots, Angiosperms, Rosids
• Order: Fabales
• Family: Caesalpinioideae
• Subfamily: Senegalia
• Genus: Senegalia
• Species: Senegalia ataxacantha
Ecology: Many butterfly species feed their larvae on the leaves, while birds including apalis, crombecs and woodhoopoes are frequently spotted catching insects from the flowers, leaves and tree stem.
Morphological characteristics Acacia ataxacantha
Bark: Bark is light to dark yellowish or grey-brown, rough, barely fissured and occasionally flaking.
Stipules: Stipules are not spinescent, arranged in groups above the nodes and are up to 12 mm x 7 mm in size and quickly become deciduous.
Prickles: Prickles up to 15 mm long that are dispersed along the internodes and are typically highly recurved, red-brown to purplish and frequently broad based.
Flower: Flowers are yellowish-white, pedicellate or seem sessile, with spike lengths ranging from 2 cm to 11 cm and peduncle lengths ranging from 0.3 cm to 2.5 cm. Usually 1/3 to 1/2 as long as the corolla, the calyx is cup-shaped, pubescent to sparingly pubescent and has lobes that are between 0.2-0.6 millimetres long on its tube. Flowers occur as a cluster which are fragment and bloom during spring and summer [5]. This species' appearance could be mistaken for Senegalia ca ra, which is distinguished by having paired prickles, stouter sections and greyish green, noticeably pendent foliage.
Leaves: petiole 1 cm to 2 cm long, adaxial gland usually present (sometimes two), variable in position, usually stalked, up to 2 mm high, sparingly to densely pubescent, rarely subglabrous, with or without recurved prickles abaxially, a gland often present at the junction of the top pair of Pinna only or between the top 1-5 and occasionally the lowest 1-3 pairs.
Seeds: Seeds olive-brown, subcircular-lenticular, 6-9 mm in diameter, compressed; areole central, small, 2.5 mm to 3 mm in diameter.
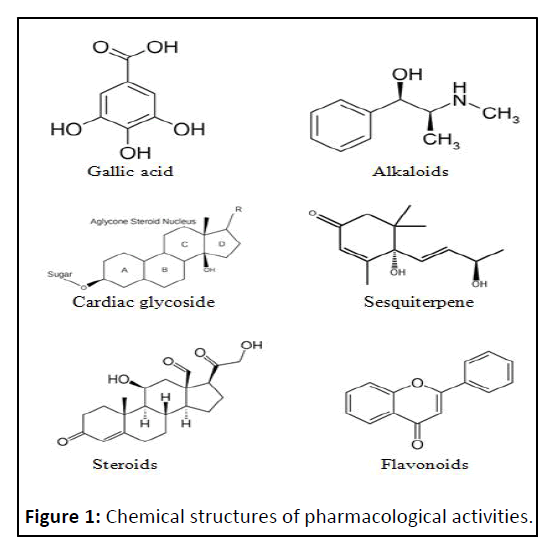
Phytoconstituents: The plant was subjected to phytochemical screening in accordance with the procedure for identifying secondary metabolites in plants. Tannins, alkaloids, flavonoids, lignans, pigments derivatives have been examined by conducting tube test method [6] (Figure 1).
Acute and chronic toxicity study
According to OECD guidelines reports, the herbal cure Acacia ataxacantha is used to treat aches, microbiological infections, ulcers, respiratory infections, mineral and vitamin supplements and dysentery. This research was done to find out how harmful Acacia ataxacantha extract in methanol was to animal experiments. The OECD criteria were followed for conducting the study on acute and chronic toxicity. The extract's oral Lethal median Dose (LD50) was calculated to be around 5000 mg/kg. At a dose of 400 mg/kg body weight, the extract significantly elevates (p˃0.05) the liver's alanine transaminase, aspartate transaminase, alkaline phosphatase and kidney's creatinine, urea and sodium ion parameters. Acute use of the extract is harmless, but repeated use may have negative effects on the liver, kidneys and stomach [7].
GIT ulcer activity using stem bark extract of Acacia ataxacantha
The goal of the study is to validate the ethno-botanical usage of ethanol extract in the treatment of gastrointestinal problems by examining the GIT profile of animals treated with it [8]. A few of the techniques used are the isolated rabbit ileum test, the ethanol/HCl-induced ulcer in rats, the rat gastrointestinal motility and charcoal screening test. At doses of 500 mg/kg and 1000 mg/kg body weight, respectively, the ethanol extract of Acacia ataxacantha stem-bark significantly (p>0.05) and dose-dependently lowers the ulcer caused by 40% and 60% when compared to the baseline. In the charcoal meal transit test, the groups pre-treated with ethanol extract showed a substantial (p>0.05) decrease in the distance travelled by charcoal meal and a noticeably increased gastro intestinal transit time. When Acacia ataxacantha stem-bark extract was administered to the rabbit ileum at concentrations of 1 mg/ ml, 10 mg/ml and 100 mg/ml, no pharmacologic effect was seen; however, at a higher dose of 200 mg/ml, a relaxation was seen on the isolated rabbit ileum test. The stem bark of Acacia ataxacantha has an anti-motility and gastroprotective action when extracted with ethanol.
Enzyme inhibitory effect
In order to determine whether the examined extracts had any inhibitory effects on certain enzymes, Acetyl Cholinesterase (AChE), amylase, glucosidase and tyrosinase were chosen. Galantamine for acetylcholinesterase, kojic acid for tyrosinase and acarbose for amylase and glucosidase were utilised as standard enzyme inhibitors for each experiment. Results were reported as these standard compounds equivalents.
Antibacterial activity
The broth microdilution method was used to test the antibacterial effects of wood extracts from Acacia ataxacantha versus Enterococcus faecalis, E. coli, Pseudomonas, Staphylococcus epidermidis and Staphylococcus aureus. Minimum Inhibitory Concentration (MIC) values for the extracts against such pathogens ranged from 0.3 mg/ml to 5 mg/ml.
Antibacterial activity the effect of extracts at 10 mg/ml on growth inhibition
The purpose of this test was to eliminate the extracts did not inhibit the growth of bacteria at 10 mg/ml. In this study, both polar as well as non-polar solvents were used for the extraction of active components from the bark of Acacia ataxacantha plant. The results show the tested bacteria are sensitive to one or more extracts. However, the dichloromethane, ethyl acetate, methanol and hydro alcoholic extracts inhibited the growth of E. faecalis. It was noticed that the ethyl acetate extract inhibited the growth of all strains tested except E. coli while dichloromethane extract inhibited P. aeruginosa. There was no evidence of bacterial growth inhibition in the hexane extract of Acacia ataxacantha (Table 1).
| Sl.no | Actions of Acacia ataxacantha |
|---|---|
| 1 | Acute and chronic toxicity study |
| 2 | Anti-microbial |
| 3 | Anti-fungal |
| 4 | Anti-diabetic |
| 5 | Anti-bacterial |
| 6 | Ulcer protective |
| 7 | Anti-inflamatory |
Table 1: Summary of actions of Acacia ataxacantha.
Antidiabetic activity
Acacia ataxacantha’s ethanolic bark extract has been shown to have anti-diabetic properties in streptozocin-induced diabetic Albino rats (Rattus norvegicus), with metformin serving as the control. In streptozotocin-induced diabetic rats, the extract at 125 mg/kg body weight had encouraging anti-diabetic effects.
Antimicrobial activity
The search for antimicrobials was focused on finding organic substances that may block Gram-positive and gram-negative bacteria, which are frequently the cause of infectious disorders. In both immunocompetent and immunocompromised hosts, gram-positive bacteria can cause a wide range of illnesses. These microorganisms continue to have a considerable impact on morbidity and death, particularly in the healthcare context, despite advances in our understanding of resistance transmission mechanisms and novel medications. The intricate and multi-layered structure of the Gram-negative cell wall creates a barrier to numerous environmental chemicals, including synthetic and natural antibiotics and restricts entry to the membrane. The findings of this study suggest that the acthaside, which was isolated from Acacia ataxacantha, may be a substance capable of penetrating this formidable barrier. Additionally, our findings indicate that the tested substance.
Ulcer protective activity
Ulcer protective activities of methanolic leaf extract of Acacia ataxacantha against indomethacin and stress induced gastric ulcer in experimental rats.
Male albino rats were stressed and given leaf extracts at doses of 100 mg/kg and 200 mg/kg body weight 30 minutes before receiving indomethacin. Rats were slaughtered, several gastrointestinal parameters including pH levels, gastric ulcer indices and gastric ulcer % inhibition were measured and ranitidine was employed as a standard antiulcer medication (Table 2).
| Sl.no | Parts of plants used | Pharmacological activity used | Types of animals used |
|---|---|---|---|
| 1 | Bark | antioxidant | C albicans (yeast) |
| 2 | leaves | Acute and chronic toxicity | Wister rat |
| 3 | Root | Antioxidant | Rat |
| 4 | Bark | Antimicrobial | S. epidermidis |
| 5 | leaves | Ulcer-protective | Rats |
Table 2: Table shows the pharmacological properties of Acacia ataxacantha.
Anti-in lammatory activity
Acacia ataxacantha leaf extracts in methanol were tested for their ability to reduce inflammation in rats paw oedema caused by carrageenan and albumin. In rats which was before with extract at doses of 200 mg/kg and 400 mg/kg body weight, the extract demonstrated important anti-inflammatory activity as evidenced by swelling of the hind paw, which progressed from time 0 to 2 hours after carrageenan injection. However, at the third hour, oedema reduction occurred in a dose-dependent circumstance and was significant only in rats. However, as compared to the normal saline, the negative control, at the fourth hour, there was a substantial difference in the development of oedema at 200 mg/kg and 400 mg/kg.
Discussion
The present review on plant demonstrates that antioxidant property is effective from the extraction of the bark. Acacia ataxacantha includes an abundance of antibacterial components that have great therapeutic effects. The leaves of Acacia ataxacantha have the acute and chronic toxicity effect. These findings may indicate that Acacia ataxacantha is a viable drug with therapeutic benefits [8]. These results of this study shows that Acacia ataxacantha may be regarded antimicrobial and laxative properties. Other unidentified components yet to be revel.
Conclusion
At least two nations Acacia ataxacantha as herbal remedy for abscesses, backaches, coughs, toothaches, headaches, malaria, pneumonia, sores and wounds and stomach issues. Acacia ataxacantha traditional medical uses for treating these ailments need to be supported by thorough in vitro and in vivo studies due to the high degree of unanimity for these diseases that has been seen. The pharmacological activities of the species, which include antibacterial, antifungal, anti-diabetic, antiinflammatory, antioxidant, laxative and ulcer-protective effects, have confirmed some of the ethnomedical uses of Acacia ataxacantha. Because of this, the pharmacological and phytochemical actions of Acacia ataxacantha support the traditional medical uses of the species against a variety of ailments, necessitating the need for thorough in vitro and in vivo studies. To determine the interaction between the separated chemicals and common antibiotics, more research is being done.
Acknowledgment
The authors express their gratitude to the principal of Bapuji pharmacy college in Davanagere, Karnataka, India, for providing the facilities needed for this work. We are also grateful to pharmacology PG students’ sushrutha K.H, A.M Geetha, Srigiri Hiremath for their suggestions and advices while writing the manuscript.
References
- Ahmadu AA, Agunu A, Lawal BA (2018) Ferulic acid ester from the from stem bark extracts of Acacia ataxacantha. Afr J Biomed Res 22:214-217
[Googlescholar] [Indexed]
- Alfred Maroyi (2018) Review of ethnopharmacology and phytochemistry of Acacia ataxacantha extract. Trop J Pharm Res 17:2301-2308
[Crossref] [Googlescholar] [Indexed]
- Amoussa AMO, Lagnika L, Sanni A (2014) Acacia ataxacantha (Bark): Chemical composition and antibacterial activity of the extract. Int J Pharm Pharm 6:138-141
- Zheleva-Dimitrova D, Sinan KI, Etienne OK, Ak G, Sharmeen JB, et al. (2021) Comprehensive chemical characterization and biological evaluation of two acacia species. (A. Nilotic and A. ataxacantha). Food Chem Toxicol 156:112446-11244
[Googlescholar] [Indexed]
- Janet M Daben, Dayil A Dashak, Olakotun Praise, Esther Ogbole, Miriam A Agba (2017) Assessment of the proximate and mineral compositions of Acacia ataxacantha leaves. Int J Sci Res Pub 6:899-900
- Kyalangalilwa B, Boatwright JS, Daru BH, Maurin O, Bank M, et al. (2013) Phylogenetic position and revised classification of Acacia s.l. (Fabaceae Mimosoideae) in Africa, including new combinations of Vachellia and Senegalia. Bot J Linn 172:500-523
[Crossref] [Googlescholar] [Indexed]
- Abbas MY, Ejiofor JI, Yakubu MI (2018) Acute and chronic toxicity profiles of methanol leaf extract of Acacia ataxacantha D.C (Leguminosae) in Wister Rats. Bull Fac Pharm 56:185-189
- Arise RO, Akapa T, Adigun MA, Yekeen AA, Oguntibeju OO (2016) Normolipidemic and antioxidant effect of ethanolic extract of Acacia ataxacantha root in streptozotocin-induced diabetic rats. Not Sci Biol 8:144-150
[Crossref] [Googlescholar] [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences