ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
A Review: Potentials of Biosurfactants in Remedying Contaminated Sites
Pushpinder Sharma* and Nivedita Sharma
Department of Basic Sciences, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Himachal Pradesh, India
- *Corresponding Author:
- Pushpinder Sharma
Department of Basic Sciences,
Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Nauni, Himachal Pradesh,
India,
Tel: 7018716646;
Email: mr.pushpindersharma28@gmail.com
Received: April 27, 2020, Manuscript No. ipjamb-20-3906; Editor assigned: April 30, 2020, PreQC No. ipjamb-20-3906(PQ); Reviewed: May 14, 2020, QC No. ipjamb-20-3906; Revised: June 28, 2022, QI No. ipjamb-20-3906; Manuscript No. ipjamb-20-3906; Published: July 26, 2022, DOI: 10.36648/2576-1412.6.6.32
Citation: Sharma P, Sharma N (2022) A Review-Potentials of Biosurfactants in Remedying Contaminated Sites. J Appl Microbiol Biochem Vol: 6 No: 6:32.
Abstract
Studies based on evaluating the influence of biosurfactants on bioremediation efficiency isconstantly growing. Biosurfactants are surface-active substances produced by diverse group of microorganisms. They are non-hazardous, biodegradable, non-toxic and environmentally friendly compounds which may be under ex-situ conditions cost effectively produced; at the site of contamination in-situ production may be stimulated and can be recovered and recycled. Due to their naturally occurring property their application in bioremediation processes may be more acceptable from a social point of view. Impressive advantages of biosurfactants include their vast structural diversity and broad range of unique properties properties, their biodegradability and possibility of cost effective production. These properties make biosurfactants a promising choice for applications in bioremediation. This paper reviews the potentials of biosurfactants in remedying contaminated sites.
Introduction
Inorganic pollutants such as heavy metals and organic pollutants like hydrophobic organic compounds cause the soil and water contamination that became a serious challenges. Cadmium (Cd), Copper (Cu), Lead (Pb), Chromium (Cr), Zinc (Zn) and Nickel (Ni) heavy metals are considered as environmental pollutants and these toxic metals accumulate in soil and water constitute potential health hazard for man and the ecosystem. HOCs (HydrophobicOrganic Compounds) such as Pentachlorophenol (PCP), Hexachlorobenzene (HCB), Polychlorinated Biphenyls (PCBs) and Dichlorophenol (DCP) are known environmental pollutants and their removal from the contaminated sites is a major environmental concern [1-3]. Besides the energy resources mainly petroleum and petroleum hydrocarbons are also major pollutants of the environment. The oil and oil products contamination may cause severe harm [4].
Literature Review
Biosurfactant have attracted much attention and their popularity seems to steadily increase during recent years. Being a frequent object of study, as work dedicated to the isolation and characterization of novel biosurfactant producers is constantly growing. Characterization of biosurfactants through a vast structural diversity and showed a broad range of properties elaborate why this group of molecules continues to entice scientific curiosity [11]. Compared to their synthetic counterparts numerous advantages of biosurfactants like biodegradability, less toxicity and eco-friendliness are yet another reason why these compounds seem so promising. Environmental friendliness combined with the ability to solubilize hydrophobic compounds may well explain why biosurfactants have also been considered as excellent compounds for enhance bioremediation of contaminated sites [12,13]. In bioremediation application of biosurfactants has been believed to be highly beneficial. Regarding the actual efficiency of biosurfactants in bioremediation is the driving force behind this manuscript, which is focused on providing a critical overview on the role of biosurfactant in bioremediation [14].
Definition of biosurfactants
Biosurfactants make for a peculiar group of compound switch exhibit notable distinction in terms of chemical structure and composition and due to this fact they have found numerous interesting applications. Various different classes, such as glycolipids, lipopeptides, lipoproteins, phospholipids, fatty acids, as wellas complex biopolymers are commonly associated with “biosurfactants”. For scientific experiments an extensively studied and reviewed group of compounds i.e rhamno lipid switch is often serves as a model biosurfactant [15]. Generally, biosurfactants exist either in an anionic or non-ionic form, however in most cases both the hydrophilic and the lipophilic part may be distinguished with relative ease. In bioremediation processes these particular characteristic is essential. Different microorganisms are capable of producing surface active compound-biosurfactants.
Pseudomonas, Bacillus, Rhodococcus and Candida genera are the most commonly indulge in the production of diverse biosurfactants. Table 1 displays a list of microorganisms that produce biosurfactants [16].
| Class/type of biosurfactant | Microorganisms |
|---|---|
| Glycolipids | |
| Rhamnolipids | Pseudomonas aeruginosa |
| Sophorolipids | Torulopsis bombicola, T. apicola |
| Trehalolipids | Rhodococcus erythropolis, Mycobacteriumsp |
| Lipopeptides and lipoproteins | |
| Peptide-lipid | Bacillus licheniformis |
| Viscosin | Pseudomonas fluorescens |
| Serrawettin | Serratia marcenscens |
| Surfactin | Bacillus subtilis |
| Subtilisin | Bacillus subtilis |
| Gramicidin | Bacillus brevis |
| Polymyxin | Bacillus polymyxia |
| Fatty acids, neutral lipids and phospholipids | |
| Fatty acid | Corynebacterium lepus |
| Neutral lipids | Nocardia erythropolis |
| Phospholipids | Thiobacillus thiooxidans |
| Fatty acid | Corynebacterium lepus |
| Polymeric surfactants | |
| Emulsan | Acinetobacter calcoaceticus |
| Biodispersan | Acinetobacter calcoaceticus |
| Liposan | Candida lipolytica |
| Carbohydrate-lipid-protein | Pseudomonas fluorescens |
| Mannan-lipid-protein | Candida tropicalis |
| Particulate surfactant | |
| Vesicles | Acinetobacter calcoaceticus |
Table 1: Main classes of biosurfactants and respective producer microorganis.
Biosurfactants contribution to bioremediation
Bioavailability of the pollutant is the main issue which directly influences the efficiency of biological treatment. Possible sorption of molecules into the soil matrix, interactions with organic matter, formation of non-aqueous phases, biotransformation and contaminant aging are the reasons of limited bioavailability, thus decreasing the efficiency of bioremediation. Enhancing the distribution of contaminants into the aqueous phase and increasing their bioavailability are the most common intended role of biosurfactants [17]. An overview of recent studies on biosurfactant assisted bioremediation was presented in Table 2.
| Type of biosurfactant | Pollutant | Relevant bioremediator | Established effect | Removal efficiency |
|---|---|---|---|---|
| Rhamnolipids | Phenanthrene | Sphingomonas sp. monoculture | Positive-solublization | 99% after 10 days compared to 84% without biosurfactant (IC-10 g/l) |
| Rhamnolipids | Anthracene | Sphingomonas sp. and Pseudomonas sp. monocultures | Positive-solublization | 52% after 18 days compared to 32% without biosurfactant for Pseudomonas (IC-25 mg/l) |
| Rhamnolipid,emulsan and indigenous biosurfactants |
Pyrene | Pseudomonas fluorescens monoculture | Positive/negative | 98% after 10 days compared to 91% without emulsan (IC-50 mg/l) |
| Rhamnolipids | Polycyclic aromatic hydrocarbons |
Alfalfa+arbuscular mycorrhizal fungi+microbial consortium of PAH degraders |
Positive-solublization | 61% after 90 days compared to 17% with only phytoremediation (IC- 12.85 g/kg of soil) |
| Sophorolipid | Hydrocarbon mixture | Autochthonous soil microflora | Positive-solubilization and mobilization |
Respectively: 95% after 2 days, 97% after 6 days and 85% after 6 days (IC-6 mg/g of soil) |
| Rhamnolipids | Diesel oil and biodiesel blends |
Microbial consortium | Positive/no effect | 77% after 7 days compared to 58% without biosurfactants for blends (IC-approx. 15 g/l) |
Table 2: An overview of recent studies on biosurfactantassisted bioremediation.
At the oil/water interface biosurfactant has tendency to deposit. Through specific interaction biosurfactants may facilitate the transport of hydrophobic contaminants (i.e., hydrocarbon-based substances) into the aqueous phase which results in solubilization and micellization. Subsequent removal of such pollutants either by soil flushing or potentially makes them more susceptible to biodegradation is due to increased mobilization [18]. Additionally, in the structure of biosurfactants heteroatoms are commonly present, in the process of forming complexes with heavy metal ions there are several active chemical groups (such as hydroxyl, carbonyl, or amine) which participate. This process enables removal of heavy metal ions and using biological methods there may enhancement of their extraction efficiency [19-21]. At the cellular membrane level biosurfactants showed strong biological activity. Enhanced hydrophobicity results from modifications, in terms of biodegradation efficiency which is considered to be relevant, or change the permeability of cellular membranes, which would potentially be beneficial during bio extraction [22].
In the actual application of biosurfactants in bioremediation processes-the molecules may either be added externally (i.e., influent, spraying, injection) or produced on-site, which seems especially promising in case of in situ treatment. In the latter case, the production of biosurfactants may be obtained by bio augmentation with appropriate microorganisms, since autochthonic microorganisms rarely exhibit satisfying efficiency [23].
Environmental contamination by oil spills and biosurfactant-enhanced remediation
Global pollution due to the release of contaminants, such as petroleum and petroleum byproducts, into the environment has become a focus of great concern both in industrialized and developing countries due to the broad environmental distribution in soil, groundwater, and air [24]. Various sources of contamination are: Accidents during fuel transportation by ships and trucks; leakage from underground storage tanks; processing during oil extraction; waste released from industrial waste that use byproducts in the process of plastic, cosmetics and pharmaceuticals. Major hydrocarbon source in the oceans comes from routines operation of ship washing natural oil leakage on the sea bed, and accidents during oil exploration and transportation [25]. Covering 163 km2 spills occurred i.e. 5943 L was the most impacting leakagein November 2011 on the Sedco 706 oil rig, operated by Chevron Brazil in Campos Bay. In the Gulf of Mexico in 2010, another largest oil spills in the world occurred due to the explosion of an oil rig off the coast of the states of Louisiana and Mississippi (USA) [26]. In the history of the United States, estimated total of three to four million barrels of oil spilled, making this the largest environmental disaster. To the ocean and coast in Dalian, China in July 2010 an oil spill of 1500 tons of crude oil caused serious environmental problems. Resident organisms are affected to the large amounts of crude oil entering the marine environment, groundwater and soil [27]. Being ahydrophobic hydrocarbon, petroleum harm the structural and functional properties of cell membranes in living organisms, and contaminate both marine and terrestrial ecosystems. The potential threat to human health posed by hydrocarbons is associated with the physical and chemical properties of these compounds, which are absorbed by the skin and quickly spread through the organism if ingested or inhaled [28-32].
Dispersal of contaminants enhanced by biosurfactantsin the aqueous phase and also increase the bioavailability of the hydrophobic substrate to microorganisms, with subsequent through biodegradation removal of such pollutants [33]. Diverse examples indicate the potential application of biosurfactants in environmental decontamination. Biosurfactant derived from Candida sphaerica removed motor oil from soil and seawater. From clay and silty soil removal rates were 75% and 92% respectively [34]. It is tested about biosurfactant from Candida tropical is which showed removal motor oil from sand with removal rates of 78% to 97% [36]. Lunasan biosurfactant derived from Candida sphaerica UCP 0995 showed removal of 95% of motor oil adsorbed to sand. Good dispersion effectiveness on crude oil shown by lipopeptides secreted by Bacillus subtilis HSO121 [35]. Lipopeptides acted effectively at dispersing oil and performed excellently at stimulating microbial oil biodegradation, which indicated its application in oil spill cleaning. The addition of both a glycolipid biosurfactant and immobilized Gordonia sp. JC11 was able to remove 60%-70% of 1 gL-1 fuel oil. Table 3 showed Biosurfactants, producing microorganisms and uses in the bioremediation of oil-contaminated environments [36-40].
| Microorganisms | Type of biosurfactant | Applications |
|---|---|---|
| Rhodococcus erythropolis 3C-9 | Glucolipid and trehalose lipid | Oil spill cleanup operations |
| Pseudomonas aeruginosa S2 | Rhamnolipid | Bioremediation of oil-contaminated sites |
| Rhodococcus sp. TW53 | Lipopeptide | Bioremediation of marine oil pollution |
| R. wratislaviensis BN38 | Glycolipid | Bioremediation applications |
| Bacillus subtilis BS5 | Lipopeptide | Bioremediation of hydrocarbon-contaminated sites |
| Pseudomonas aeruginosa BS20 | Rhamnolipid | Bioremediation of hydrocarbon-contaminated sites |
| Micrococcus luteus BN56 | Trehaloset etraester | Bioremediation of oil-contaminated environments |
| Nocardiopsis alba MSA10 | Lipopeptide | Bioremediation |
| Pseudomonas alcaligenes | Rhamnolipid | Environmental applications |
| C. lipolytica UCP0988 | Sophorolipids | Oil recovery |
| C. guilliermondii UCP0992 | Glycolipid complex | Removal of petroleum derivate motor oil from sand |
| C. sphaerica UCP0995 | Protein-carboydrate-lipid complex | Oil removal |
| C. lipolytica UCP0988 | Sophorolipids | Removal of petroleum and motor oil adsorbed to sand |
| C. glabrata UCP1002 | Protein-carboydrate-lipid complex | Oil removal |
| Pseudozyma hubeien sis | Glycolipid | Bioremediation of marine oil pollution |
| Pseudomonas cepacia CCT6659 | Rhamnolipid | Bioremediation of marine and soil environments |
Table 3: Biosurfactants, producing microorganisms and uses in the bioremediation of oil-contaminated environments.
Discussion
Application in Microbial Enhanced Oil Recovery (MEOR)
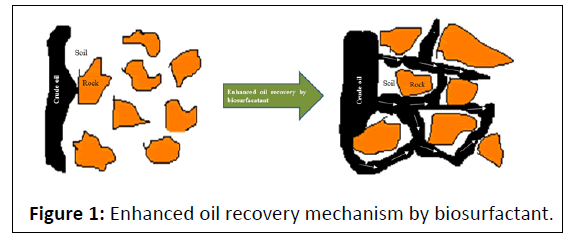
Oil spills cause devastating effect on aquatic life on marine environment. Chemically synthesized surfactants had been reported for their toxicity on aquatic organisms, so were, treated them unsuitable for remediation [41,42]. One of the inherent alternatives for this purpose wastofind the biomolecules which had surface activity as well as the emulsifying activity along with the low Critical Micelle Concentration (CMC) characteristics. Due to the high cost of chemical tension active agents hinders the widespread use of surfactants in oil recovery processes [43]. Thus, to reduce the interfacial tension between oil/water and oil/rock, biosurfactants have been employed which leads to a reduction in the capillary forces that impede oil from moving through rock pores (Figure 1). The biosurfactants emulsify the hydrocarbons in water to form various mixtures and make them water soluble. Lichenysins, rhamnolipids and surfactin are the few surfactants which are found to be successful in the remediation of the oil contamination [44-46]. Kim et al. isolated a bacterium from a crude oil sample which produced a biosurfactant that had good emulsifying properties on crude oil and paraffin [29]. Literature suggested that biosurfactants produced from marine bacterium were capable enough to destroy the oil slicks which float on the surface of water in order to promote the dispersion of oil in water by forming a stable emulsion thereby enhancing the rate of biodegradation. Due to these factors, biosurfactants had shown potential in its applications of cleaning up the oil spills on shorelines and in the sea [47-50].
The ubiquitous presence of the marine bacteria which degrade hydrocarbons have been recognized as hydrocarbonoclastic bacteria [51]. These bacteria degrade the hydrocarbons present in the polluted sites of marine environments [2]. Different studies revealed by that the mixture of the biosurfactants stimulated the degradation of hydrocarbons in the marine environment [52,53]. Hydrocarbonoclasticity bacterial consortium has a wide range of degradation capabilities on both aliphatic as well as aromatic fractions of crude oil. In general, biosurfactants produced by oil degrading bacteria can enhance the assimilation of the hydrocarbons as well as the nutrients available in the environment. Emulsifying agents synthesize by some groups of microorganisms, hence emulsifiers have been used for cleaning up the oil as they help in hydrocarbon degradation. In the industrial scale biosurfactants can be largely produced by fermentation process; Lichenysins were produced from B. licheniformis JF-2, Lichenysin even at lower concentrations (10-60 mg/l) was able to reduce the surface tension between the interfacial surfaces into ultra lesser values (10-2 mN/m) [54,55]. The range of temperature (≤ 140°C), pH (6-10), and salinity (up to 10% w/v NaCl) variation had no effect on its activity. By altering the wettability capacity of the porous media biosurfactant adsorbs the oil. Acinetobact ervenetianus ATCC 31012 produce an emulsion which at 0.1 mg/ml conc. removes 89% of crude oil which had been reabsorbed to the samples of limestone and at 0.5 mg/ml concentration 98% of removal was achieved [56].
Studies had emphasized on the possibility of introducing the bacteria which produce biosurfactants in to the infected sites, so that they can utilize the nutrients present in the oil well for their growth, but it was more suitable for the strategy of microbially enhanced oil recovery where the bacteria would metabolically active even at extreme conditions in the petroleum reservoirs [57]. Many bacterial species that produce biosurfactants had been described for the microbially enhanced oil recovery in-situ applications that belong to Bacillus sp. because of their thermal and halotolerance ability. A typical Bacillus strain was grown and produced lichenysin by both anaerobic and aerobic processes at relatively high temperatures ranging from 40°C-60°C. Different processes can be approached to exploit the biosurfactant producing strains in oil recovery applications [58].
Application of biosurfactants in removing heavy metal ions
Biosurfactant-assisted removal of heavy metal ions by complex formation and subsequent mobilization has received much attention. Heavy metals are the inorganic pollutants that pose the greatest potential risk to human health occurs naturally in rock, soil, plants and animals. Ions dissolved in water, vapour or minerals in rock, sand and soil are the different forms of metals. Metals can also be bonded to organic or inorganic molecules or trapped by air particles. Metals release into the air and water by both natural and anthropogenic processes. Due to number of industrial activities, such as mining, metal forging, the manufacturing of automobile batteries, emissions from vehicles and industrial waste dumps and the dispersion of ash from incineration processes heavy metal contamination occurs [59]. Heavy metals in soil causes serious problems, as they are non-biodegradable, which causes the contamination of biological systems and the subsoil through the process of lixiviation. For instance, lead is found in 15% of contaminated lands in the USA, followed by chrome, cadmium and copper, which are found in 7% to 11% of soils. In the treatment of soil contaminated by heavy metals numerous methods have been developed and implemented. To treat contaminated soil two major technologies are employed. In the first technology there is immobilization of heavy metals in a solid matrix strongly bonded to the soil to minimize migration [60]. The second technology promotes the mobility of the metal and through desorption and solubilisation its migration to a liquid phase. Most widely applied methods includes the flushing of soil with acids and chelating agents, such as Ethylene Diamine Tetraacetic Acid (EDTA). Flushing with acids reduces the fertility of the soil and leads to changes in the physicochemical composition (Reed et al.) [47]. Moreover, the use of EDTA is not good from the safety point of view due to its low degradation rate. The difficulty in recovering the heavy metal from the metal-EDTA complex also prohibits the use of this method. For the remediation of soils contaminated by heavy metals and oils the use of surfactants is a potential and permanent solution. In solutions surfactants allow the reuse of the soil by facilitating the solubilisation, dispersion and desorption of the contaminants. In decontamination tests a number of synthetic surfactants have been evaluated. However, the need to replace synthetic compounds with surface active compounds of microbial originhas led to research into the usage of potential biosurfactants. In this respect, studies showed the potential of rhamnolipids, sophorolipids (both of a bacterial origin) and surfactin [61]. The biodegradability, ionic nature, low degree of toxicity and excellent surface properties make biosurfactants promising compounds for the removal of heavy metals from soil and sediment.
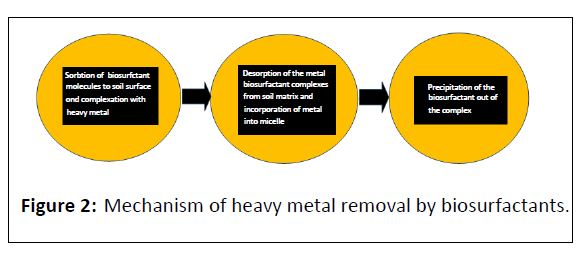
The removal of metals by ionic biosurfactants is through three steps occur in the order (Figure 2). According to Mulligan, with different concentrations of biosurfactants removal is possible [10]. Das et al. report that the removal of cadmium using an aqueous solution also occurred at concentrations below the CMC, while a concentration fivefold greater than the CMC resulted in the nearly complete removal of 100 ppm of metallic ions [15]. Wen et al. studied the degradation of a rhamnolipid in soils contaminated by cadmium and zinc and found that this compound could remain in the soil long enough to enhance the phytoextraction of the metal [57]. Through electrostatic interactions heavy metals are trapped within the micelles and through precipitation or membrane separation methods it can be easily recovered. With metal through ionic bonds anionic biosurfactants create non-ionic complexes. Such bonds are stronger as compare to those which established between the soil and metal [62]. Due to the reduction in interfacial tension the metal-biosurfactant complex is desorbed from the soil matrix. Cationic biosurfactants can replace similarly charged metal ions through competition for some, but not all, the negatively charged surfaces (ion exchange). By surfactant micelles metal ions can also be removed from the soil surface [63]. Biosurfactants offer indisputable advantages, since microorganisms capable of producing surfactant compounds do not need to have the ability to survive in the soil contaminated by a heavy metal, although the continuous addition of biosurfactant is required in the process. Biosurfactants have also been applied in mining. As compare to conventional chemical reagents, Pseudomonas sp. and Alcaligenes sp. produced tensioactive compounds which have been used for the floatation and separation of scheelite and calcite, with recovery rates of 95% for CaWO4 and 30% for CaCO3. Biosurfactants produced by species of candida have been successfully employed in the floatation of heavy metals, demonstrating the ability to remove more than 90% of cations from columns and in dissolved air floatation processes. An anionic polysaccharide produced by Acinetobactercalcoaceticus A2, denominated biodispersan, has been used for the prevention of flocculation and dispersion in mixture of calcareous rock and water. Rhamnolipid derived from Pseudomonas Aeruginosa capable of the removal of chromium containing precipitates [64]. A yeast biosurfactant derived from Candida sphaerica was first evaluated in removing heavy metals in soil. There was removal of 95, 90 and 79% Fe, Zn and Pb, respectively. And also removed the 75% Pb and 87% Cd from aqueous solution. Saponin, rhamnolipid, and sophorolipid can effectively enhance heavy metals removal from the sludge in the electrokinetic tests. Biosurfactant rhamnolipid showed the removal of Cu (80.21%), Cd (86.87%), Pb (63.54%) and Cr (63.54%) by 0.8% rhamnolipid after 12 h at pH 7.0.
Advantages of using biosurfactantin bioremediation: Biosurfactants are readily biodegradable and therefore do not constitute additional pollution threat. Biosurfactants reduce the total time taken for biodegradation of PAHs in contaminated soils. Biosurfactants reduce surface and interfacial tension, thereby increasing the surface areas of insoluble compounds leading to increased mobility and bioavailability of hydrocarbons. Surface active compounds produced by bacterial strains do not need to have survival ability in soils contaminated with heavy metals. They are environmentally friendly, less toxic and non-hazardous. Their production is potentially less expensive than synthetic surfactants and is achievable in situ at the contaminated sites from inexpensive raw materials [65].
Disadvantages of using biosurfactant in bioremediation: There is a relatively high production and recovery cost, as well as the difficulty of their mass production. Prolonged exposure of skin to biosurfactants can cause chafing because surfactants (like soaps) disrupt the lipid coating that protects the skin and other cells.
Conclusion
In bioremediation processes the application of biosurfactants is currently an ambiguous topic. Biosurfactants have shown their potential for remediation of contaminated sites by increasing biodegradation rate and reducing contaminant minimum concentration. Soil and water that are contaminated with organic and inorganic pollutants can be effectively treated with biosurfactants. Careful and controlled use of biosurfactants will help to enhance cleanup of toxic environmental pollutants and render the environment clean.
References
- Eruke OS, Udoh AJ (2015) Potentials for biosurfactant enhanced bioremediation of hydrocarbon contaminated soil and water-a review. Adv Res 4: 1-14.
- Karlapudi AP, Venkateswarulu TC, Tammineedi J, Kanumuri L, Ravuru BK, et al. (2018) Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 4: 241-249.
- Pacwa-Plociniczak M, Plaza GA, Piotrowska-Seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: Recent advances. Int J Mole Sci 12: 633-654.
- Kumar A, Bisht BS, Joshi VD, Dhewa T (2011) Review on bioremediation of polluted environment: A management tool. Int J Environ Sci 1: 1079.
- Shavandi M, Mohebali G, Haddadi A, Shakarami H, Nuhi A (2011) Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloid Surfaces B: Biointerfaces 82: 477-482.
- Zheng C, Li Z, Su J, Zhang R, Liu C, et al. (2012) Characterization and emulsifying property of a novel bioemulsifier by Aeribacillus pallidus YM‐1. J Appli Microbiol 113: 44-51.
- Luna JM, Rufino RD, Sarubbo LA, Campos-Takaki GM (2013) Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surfaces B: Biointerfaces 102: 202-209.
- Marchant R, Banat IM (2012) Biosurfactants: A sustainable replacement for chemical surfactants?. Biotechnol Lett 34: 1597-1605.
- Makkar RS, Rockne KJ (2003) Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbons. Environ Toxicol Chem 22: 2280-2292.
- Mulligan CN (2009) Recent advances in the environmental applications of biosurfactants. Curr Opinion Colloid Interface Sci 14: 372-378.
- Kosaric N (2001) Biosurfactants and their application for soil bioremediation. Food Technol Biotechnol 39: 295-304.
- Lawniczak L, Marecik R, Chrzanowski L (2013) Contributions of biosurfactants to natural or induced bioremediation. Appl Microbiol Biotechnol 97: 2327-2339.
- Ron EZ, Rosenberg E (2001) Natural roles of biosurfactants: Mini review. Environ Microbiol 3: 229-236.
- Rahman PK, Gakpe E (2008) Production, characterisation and applications of biosurfactants-Review. Biotechnology 7: 360-370.
- Mukherjee AK, Das K (2010) Microbial surfactants and their potential applications: An overview. Biosurfactants 672: 54-64.
- Soberon-Chavez G, Lepine F, Deziel E (2005) Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 68: 718-725.
- Abdel-Mawgoud AM, Lépine F, Déziel E (2010) Rhamnolipids: Diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86: 1323-1336.
- Rahman KSM, Banat IM, Thahira J, Thayumanavan T, Lakshmanaperumalsamy P (2002) Bioremediation of gasoline contaminated soil by a bacterial consortium amended with poultry litter, coir pith and rhamnolipid biosurfactant. Bioresour Technol 81: 25-32.
- Gorna, H, Lawniczak L, Zgola-Grzeskowiak A, Kaczorek E (2011) Differences and dynamic changes in the cell surface properties of three Pseudomonas aeruginosa strains isolated from petroleum-polluted soil as a response to various carbon sources and the external addition of rhamnolipids. Bioresour Technol 102: 3028-3033.
- Allard AS, Neilson AH (1997) Bioremediation of organic waste sites: A critical review of microbiological aspects. Int Biodeter Biodegrad 39: 253-285.
- Maier RM, Soberon-Chavez G (2000) Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl Microbiol Biotechnol 54: 625-633.
- Ochoa‐Loza FJ, Artiola JF, Maier RM (2001) Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J Environ Quality 30: 479-485.
- Asci Y, Nurbas M, Acikel YS (2008) Removal of zinc ions from a soil component Na-feldspar by a rhamnolipid biosurfactant. Desalination 223: 361-365.
- Johnsen AR, Karlson U (2004) Evaluation of bacterial strategies to promote the bioavailability of polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol 63: 452-459.
- Xiao-Hong PEI, Xin-Hua ZHAN, Shi-Mei WANG, Yu-Suo LIN, Li-Xiang ZHOU (2010) Effects of a biosurfactant and a synthetic surfactant on phenanthrene degradation by a Sphingomonas strain. Pedosphere 20: 771-779.
- Cui CZ, Zeng C, Wan X, Chen D, Zhang JY, et al. (2008) Effect of rhamnolipids on degradation of anthracene by two newly isolated strains, Sphingomonas sp. 12A and Pseudomonas sp. 12B. J Microbiol Biotechnol 18: 63-66.
- Husain S (2008) Effect of surfactants on pyrene degradation by Pseudomonas fluorescens 29L. World J Microbiol Biotechnol 24: 2411-2419.
- Zhang J, Yin R, Lin X, Liu W, Chen R, et al. (2010) Interactive effect of biosurfactant and microorganism to enhance phytoremediation for removal of aged polycyclic aromatic hydrocarbons from contaminated soils. J Health Sci 56: 257-266.
- Kang SW, Kim YB, Shin JD, Kim EK (2010) Enhanced biodegradation of hydrocarbons in soil by microbial biosurfactant, sophorolipid. Appl Biochem Biotechnol 160: 780-790.
- Owsianiak M, Chrzanowski L, Szulc A, Staniewski J, Olszanowski A, et al. (2009) Biodegradation of diesel/biodiesel blends by a consortium of hydrocarbon degraders: Effect of the type of blend and the addition of biosurfactants. Bioresour Technol 100: 1497-1500.
- Owsianiak M, Szulc A, Chrzanowski L, Cyplik P, Bogacki M, et al. (2009) Biodegradation and surfactant-mediated biodegradation of diesel fuel by 218 microbial consortia are not correlated to cell surface hydrophobicity. Appl Microbiol Biotechnol 84: 545-553.
- Lebrero R, Estrada JM, Munoz R, Quijano G (2012) Toluene mass transfer characterization in a biotrickling filter. Biochem Eng J 60: 44-49.
- Souza EC, Vessoni-Penna TC, de Souza Oliveira RP (2014) Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int Biodeter Biodegrad 89: 88-94.
- Costa AS, Romao LPC, Araujo BR, Lucas SCO, Maciel STA, et al. (2012) Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresource Technol 105: 31-39.
- Bachmann RT, Johnson AC, Edyvean RG (2014) Biotechnology in the petroleum industry: An overview. Int Biodeter Biodegrad 86: 225-237.
- Batista RM, Rufino RD, Luna JM, de Souza JEG, Sarubbo LA (2010) Effect of medium components on the production of a biosurfactant from Candida tropicalis applied to the removal of hydrophobic contaminants in soil. Water Environ Res 82: 418-425.
- Feng JQ, Gang HZ, Li DS, Liu JF, Yang SZ, et al. (2019) Characterization of biosurfactant lipopeptide and its performance evaluation for oil-spill remediation. RSC Adv 9: 9629-9632.
- Laorrattanasak S, Rongsayamanont W, Khondee N, Paorach N, Soonglerdsongpha S, et al. (2016) Production and application of Gordonia westfalica GY40 biosurfactant for remediation of fuel oil spill. Water Air Soil Pollut 227: 1-13.
- Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2016) Biosurfactants: Multifunctional biomolecules of the 21st century. Int J Mole Scie 17: 401.
- Kim P, Oh DK, Kim SY, Kim JH (1997) Relationship between emulsifying activity and carbohydrate backbone structure of emulsan from Acinetobacter calcoaceticus RAG-1. Biotechnol Lett 19: 457-459.
- Bach H, Berdichevsky Y, Gutnick D (2003) An exocellular protein from the oil-degrading microbe Acinetobacter venetianus RAG-1 enhances the emulsifying activity of the polymeric bioemulsifier emulsan. Appl Environ Microbiol 69: 2608-2615.
- Yakimov MM, Timmis KN, Wray V, Fredrickson HL (1995) Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl Environ Microbiol 61: 1706-1713.
- Juwarkar AA, Nair A, Dubey KV, Singh SK, Devotta S (2007) Biosurfactant technology for remediation of cadmium and lead contaminated soils. Chemosphere 68: 1996-2002.
- Hong KJ, Tokunaga S, Kajiuchi T (2002) Evaluation of remediation process with plant-derived biosurfactant for recovery of heavy metals from contaminated soils. Chemosphe 49: 379-387.
- Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. J Envl Manag 92: 2355-2388.
- Peng JF, Song YH, Yuan P, Cui XY, Qiu GL, et al. (2002) The remediation of heavy metals contaminated sediment. J Ha Mat 161: 633-640.
- Reed BE, Carriere PC, Moore RJ (1996) Flushing of a pb (II)contaminated soil using HCL, EDTA and CaCl2. J Env 122: 48-50.
- Asçi Y, Nurbas M, Acikel YS (2008) A comparative study for thesorption of Cd (II) by soils with different clay contents and mineralogy and the recovery of Cd(II) using rhamnolipid biosurfactant. J Haz Mat 154: 663-673.
- Mulligan CN (2009) Recent advances in the environmental applicationsof biosurfactants. Curr Opin Coll Inter Sci 14: 372-378.
[Crossref][Google Scholar][Pubmed]
- Dahrazma B, Mulligan CN (2007) Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration. Chemo 69: 705-711.
- Herman DC, Artiola JF, Miller RM (1995) Removal of cadmium, lead and zinc from soil by a rhamnolipid biosurfactant. Env Sci Technol 29: 2280-2285.
- Mulligan CN (2009) Recent advances in the environmental applicationsof biosurfactants. Curr Opi Coll Inter Sci 14: 372-378.
- Neilson JW, Artiola JF, Maier RM (2003) Characterization of lead removal from contaminated soils by non-toxic soil-washing agents. J Env Quali 32: 899-908.
- Ochoa-Loza FJ, Noordman WH, Jannsen DB, Brusseau ML, Maier RM, et al. (2007) Effect of clays, metal oxides, and organic matter on rhaminolipid biosurfactant sorpition by soil. Chemosphere 66: 1634-1642.
- Tan HM, Champion JT, Artiola JF, Brusseau ML, Miller RM, et al. (1994) Complexation of cadmium by a rhamnolipid biosurfactant. Env Sci Technol 12: 2402-2406.
- Das P, Mukherjee S, Sen R (2009) Antiadhesive action of a marinemicrobial surfactant. Colloids Surfaces B: Biointerfaces 71: 183-186.
- Wen J, Stacey SP, McLaughlin MJ, Kirby JK (2009) Biodegradationof rhamnolipid, EDTA and citric acid in cadmium and zinc contaminated soils. Soil Biol Biochem 41: 2214-2221.
- Kitamoto D, Isoda H, Nakahara T (2002) Functions and potential applications of glycolipid biosurfactants: From energy-saving materials togene delivery carriers. J Bio 94: 187-201.
- Pacwa-Plociniczak M, Plaza GA, Piotrowska Seget Z, Cameotra SS (2001) Environmental applications of biosurfactants: Recent advances. Int J Mol Sci 13: 633-654.
- Nitschke M, Pastore GM (2002) Biosurfactantes: Propriedadeseaplicacoes. Quimica Nova 25: 772-776.
- Menezes CTB, Barros EC, Rufino RD, Luna JM, Sarubbo LA, et al. (2010) Replacing synthetic with microbial surfactants as collectors in the treatment of aqueous effluent produced by acid mine drainage, using the dissolved air flotation technique. App Biochem Biotechnol 163: 540-546.
- Albuquerque CF, Luna Finkler CL, Rufino RD, Luna JM, Menezes CTB, et al. Evaluation of biosurfactantsfor removal of heavy metal ions from aqueous effluent using flotation techniques. Int Review Chem Eng 4: 156-161.
- Abyaneh AS, Fazaelipoor MH (2016) Evaluation of rhamnolipid (RL) as abiosurfactant for the removal of chromium from aqueous solutions by precipitate flotation. J Environ Manage 165:184-187.
- Tang J, He J, Liu T, Xin X ( 2017) Removal of heavy metals with sequential sludge washing techniques using saponin: optimization conditions, kinetics, removal effectiveness, binding intensity, mobility and mechanism. RSC Adv 7: 33385-33401.
- Chen W, Qu Y, Xu Z, He F, Chen Z, et al. (2017) Heavy metal (Cu, Cd, Pb, Cr) washing from river sediment using biosurfactant rhamnolipid. Environ Sci Pollut Res 19: 16344-16350.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences