ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
A Review on Cadmium Contamination in Soil and Bioaccumulation by Tobacco, its Source, Toxicity and Health Risk
Angelique Iradukunda1,2, Dan Zhang1*, Ram Proshad1,2, Philbert Mperejekumana3
1Institute of Mountain Hazards and Environment, Chinese Academy of Sciences, Sichuan, China
Abstract
Cadmium (Cd) can be regarded as hyperactive metal that is accumulated by tobacco plants from the soil and is associated with several health hazards posed to tobacco consumers, as well as affect environmental quality specifically soil heath. The present review emphasizes mechanisms of uptake and accumulation of cadmium from the soil by the tobacco plant, its sources, toxicity, and health hazards caused by it. As an overall outcome, the present study provides a significant idea of a soil-metal-tobacco relationship, which is a useful tool to provide information that leads to limited accumulation and consumption of Cd within the ecosystem. Besides, absorption of Cd from soil solution and its translocation from roots to upper shoots involved moving cadmium from the soil to tobacco plants. The absorption is driven by soil, environmental, and plant factors, while the translocation is enhanced by tobacco physiological function. Thereafter, researchers should focus on the efficiency of the tobacco plant in the phytoremediation of Cd polluted soil.
Keywords
Cadmium; Tobacco; Bioaccumulation; Soil: Health risk
Introduction
The economic development of humankind has brought various innovative ideas like industrialization that have been accelerated in the nineteenth century and intensified agriculture that aimed to increase production by using advanced techniques such as the application of sewage sludge, pesticides, lime, irrigation waters and fertilizers. Those practices together with other anthropogenic activities like mining and transportation means have been recognized as the primary source of environmental contaminants [1]. Among contaminants, cadmium and other toxic metals can directly enter the human body through consumption of contaminated water or indirectly through their bioaccumulation in the food chain [2]. Besides, cadmium absorbed through dermal absorption, may be harmful to human body. Cadmium is of great concern in this study as it is quickly transferred and accumulated by plants, and can travel long distances from the source of emission by atmospheric transport [3,4]. Cadmium is not an essential element and its uptake increases with the increase in its concentration in soil [5]. Moreover, various organizations have mentioned cadmium as a potentially toxic metal in the ecosystem [6].
Cadmium can be very toxic even in low concentrations; as a result, high Cd concentration can have toxic effects on soil organisms and is considered a primary soil pollutant [7]. It can be released in soil, water, and atmosphere then spread into organisms either through consumption of contaminated plants or water and smokes. This metal can be a threat to soil microbial activity by poisoning the earthworms and other soil organisms, which implies that a high concentration of cadmium in soil may affect soil structure particularly and whole soil ecosystem [8]. In addition, cadmium can quickly transfer into vegetative cover and ultimately enter the food chain as a higher potential carcinogenic metal [7]. Its bioaccumulation is highly associated with human health effects, which implies vulnerability to various forms of diseases, including lung cancer [9]. Its biological half-life in humans of 10-35 years may lead to renal tubular dysfunction; High intake of cadmium can lead to disturbances in calcium metabolism, the formation of kidney stones and effects on bone [10].
On the other hand, Tobacco is a popular plant known to accumulate a considerable quantity of toxic metals including Cd, which can be concentrated at a level of 0.5 to 5 mg/Kg Cd in tobacco leaves [11]. Moreover, various literatures have mentioned the accumulation of cadmium in tobacco and uptake of metals from the soil by tobacco plant [12,13]. Cadmium can particularly be accumulated in leaves. In different regions of the world, bioaccumulation of metals with cadmium has serious concerns as the accumulation of Cd in tobacco affect smokers and non-smokers as it has been proven that there are various health effects faced by cigarettes’ smokers in different regions of the world [14]. It was observed in Pakistan that the most used cigarette types were reported to have a massive concentration of heavy metals that may be increased by tobacco growth and production conditions [15]. In Nigeria, a higher level of metal concentration was reported in tobacco leaves, which are the most usable part of tobacco; generally, the level of metals is attributed to the physicochemical properties of soil [16]. According to the available literature, the future projections of 2002 to 2030 predict that the annual mortality rate resulting from tobacco-caused diseases will increase in middle and low-income countries. Various studies revealed those harmful effects of tobacco to not only Nicotine but also to different toxic metals contamination in tobacco leaves [11,14].
However, there are much fewer studies carried out specifically for soil-cadmium-tobacco relation. Furthermore, some ways and mechanisms of cadmium accumulation have been discussed but detailed discussions are needed to provide significant facts about its bioaccumulation from the soil by tobacco [17,18]. Although an increase in Cd contamination and its presence in tobacco has been recorded over the past years, still there is a need of understanding how Cd transfer in shoots of tobacco and accumulates in its leaves from soil. There is still a need to upsurge the understanding of the mechanism within soil-cadmium-tobacco relation. Therefore, to fill this gap, the present study emphasizes on Cd contamination on soil, its sources, and toxicity as well as its effects on the environment and discussed the potentials of tobacco in phytoaccumulation of soil cadmium.
Cadmium Sources to Invade Soil
There has been a worldwide trend in Cd production since the 1920s, which fluctuated around 20,000 tons since 1987, and it was 24,900 metric tons in the last year of 2019 [19,20]. This increase may be attributed to different sources, either geologic or anthropogenic like agricultural activities, mining, transportation and industries. Soil Cd may appear in the field as a result of soil parent material; however, its concentration can be increased by the disposal of substances in the soil environment like sewage sludge and fertilizers and other human activities [21]. Mean values of cadmium in igneous, metamorphic and sedimentary rocks range from 0.02 to 25 mg/kg-1, although it can reach higher in phosphorite marine sedimental rocks [22]. The report on chemical contaminants on the United States Department of Energy (DOE) showed the range of cadmium in soils to varying from 0.01mg/kg to 345mg/kg [23]. At the same time, literature reports the highest total Cd concentrations in Histosols (organic soils) and the lowest in highly weathered soils (Ultisols and Alfisols) [22]. In China, 450 ton of Cd was released into the atmosphere from the zinc smelting activities from 1989 to 2001, which increased up to 743 metric tons in 2009, and in 2015, a large part of agricultural soils in china was Cd polluted [24,25]. Soil chemical properties are the key determinants of tobacco contents [26]. At the same time, some external factors like fertilization and irrigation may enhance its growth; however, they may be a source of some unnecessary elements.
The potential of agricultural activities in a release on cadmium and other metals in the soil is mainly observed through the use of fertilizers, and pesticides. It can also be through the use of contaminated water in an irrigation system. In the recent research, the use of both organic and inorganic fertilizers (mainly calcium and phosphate fertilizers) was said to be accompanied by some additional quantities of metals in the field [27]. A study conducted in Hebei province, China showed that the accumulation of Cd in the soil as a result of agricultural activities like irrigation water and fertilizers that were applied in the field [6]. Phosphate fertilizers are the most mentioned fertilizer in many research to be a potential source of cadmium in soil [7,28,29]. However, Bozhinova reported a less significant effect of long-term phosphorus fertilizer application on cadmium status in soil and tobacco [30]. Generally, phosphates can accumulate heavy metals, and while making phosphate fertilizers, phosphate rocks are used as raw materials, so that the contained metals will be exposed to the users of these products. Besides, sewage sludge is usually used in irrigation is also a source of heavy metal, including cadmium, which is the most burning question at present time. Metals in sludge may be in various forms like exchangeable, adsorbed, precipitated or combined with carbonates or sulfates, which support the concentration of Cd and other metals reported having increased in plants part due to the addition of sludge on soil [31].
Furthermore, during hot furnace processing (greater than its boiling point of 7650C), Cd can also be volatilized to become airborne as long as forming oxides in the environment [32]. The use of Cd in Nickel/Cadmium batteries of good quality and high tolerance to physical and electrical stress [32]. This type of raw material in industrial processing can ensure the direct release of Cd particles in the soil environment through industrial solid and liquid wastes disposal. Besides, after investigation of the distribution of heavy metals in soils around industrial sites of Dongguan in China, the level of Cd and other heavy metals were elevated in the vicinity of industrial sites of that region [33].
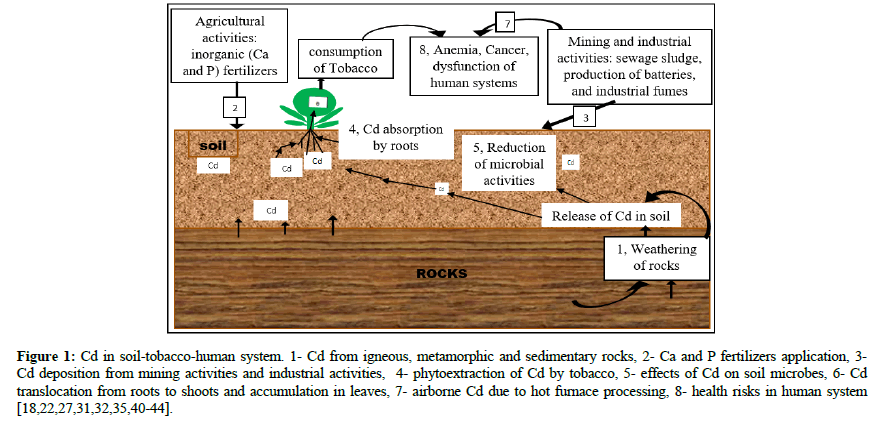
Besides, Various literature presents mining and industrial activities as an alternative source of Cd toxicity [22,34]. Especially, abandoned mines and tailings are the pronounced source of heavy metals in mining areas [35]. In a gold mining region in east Africa, a higher level of cadmium was observed in soil, which is more significant than the standard one of 0.01-2.5 mg/kg-1 Cd in unpolluted soil and there was a significant concentration of this metal in the rivers and ponds surrounding the region [36,37]. In West Africa, several findings report influence on increasing heavy metal concentration in near mining areas; in southwestern Nigeria, Cd concentration increased by 54% in a period of ten years starting from 2003 [38]. In Ghana, the values observed for Cd concentration in boreholes that were nearly closer to WHO limit values that could affect small-scale gold mining in that region [39]. Briefly, many different anthropogenic activities are major sources of contaminants as they release strange materials in the natural environment. Heavy metals can also come from motor vehicle and vehicle exhaust emissions [27]. Where, Figure 1 summarizes all the described sources of Cd and its corresponding effects on soil and human health [40-44].
Figure 1: Cd in soil-tobacco-human system. 1- Cd from igneous, metamorphic and sedimentary rocks, 2- Ca and P fertilizers application, 3- Cd deposition from mining activities and industrial activities, 4- phytoextraction of Cd by tobacco, 5- effects of Cd on soil microbes, 6- Cd translocation from roots to shoots and accumulation in leaves, 7- airborne Cd due to hot furnace processing, 8- health risks in human system [18,22,27,31,32,35,40-44].
Absorption of Cadmium by Tobacco
The transfer of Cd from soil to plants depends on soil, plant and metal properties [45]. The past study consistently reported the water-soluble and the exchangeable fractions as the most mobile and bioavailable forms of heavy metals [46]. The availability of Cd along with its absorption by tobacco largely depends on its types of binding forms, that heavy metals in uncontaminated soils and sediments are mainly immobile as they are bound to silicates and primary minerals [47]. However, the mobility of Cd occurs mainly when the metals are in the aqueous phase, in a dissolved form or associated with colloidal particles [48]. Additionally, various soil factors such as soil solution pH, redox potential, clay content, soil texture, iron and manganese oxide, organic matter content, and the presence of other positive cations in the soils affect Cd and other metals mobility and availability to plant [49]. Those soil parameters are significant to determine the availability of metal in soil solution.
Availability of Cd is likely to increase as the soil pH decreases [50]. It also has a positive correlation with organic matter known to use its organic acids and compounds to chelate metals to increase their availability, and in the presence of organic matter, there is an increase in Cation Exchange Capacity (CEC) usually indicated by higher sorption site due to raised number of negative charges in the soil as organic matter and Cation Exchange Capacity act as an indicator of metal availability in soil [51]. A particular conclusion was drawn that iron (Fe) fraction increased cadmium availability in paddy field and that Fe plaque can act as cadmium sequester, which significantly supports the importance of reduction-oxidation activity in paddy soil as a result of waterlogging and drying cycle in wetlands [51]. Cadmium availability is higher in reducing (flooded) and lowers in the oxidizing field [52]. Therefore, Soil pH and actual Cd concentration in the field is recognized as significant main soil conditions that influence cadmium availability and its absorption by tobacco plants. On the other hand CEC, the content of oxides of Fe and Mn, and interactive effects with other elements (Zn, Cu, Ni, Mn, Se, P), and soil temperature are the minor factors on cadmium activity in soil [51].
Cadmium and other metal’s bioavailability and absorption can also be increased by the plants themselves [53]. Like hytosiderophores, nicotianamine and organic acids are a few examples of chelating compounds that are released by roots and might influence metal uptake [42]. In addition, some soil amendments are known to have a significant influence on cadmium availability. Silicon addition to soil results to increased residual Cd but decreased available Cd, and it enhances the increase of soil pH, which is realized to have a negative correlation with Cd and other metals availability [54]. Organic amendments do not influence total Cd content but decrease Cd availability by increasing adsorption or complexation and precipitation of metal. Biochar affects Cd availability by increasing the sorption site while liming make precipitation together with changing pH towards alkalinity [19]. The addition of humic acid resulted in the decreased level of metals available to tobacco plants [55]. In the other study, zeolite was applied in Cd contaminated soil and it was concluded that this amendment can reduce Cd availability in tobacco, as the amount of Cd in tobacco parts decreased as the level of zeolite was increased [57].
Potentials of Tobacco in Phytoaccumulation of Cadmium
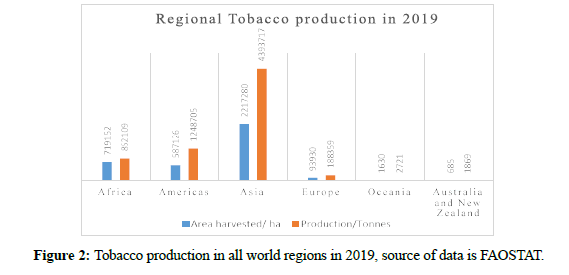
Tobacco is an industrial cash crop in many countries, where it has a significant quantity of exported harvest [57]. Its production and commercialization started in recent years, in which its production increased to over six million tons produced worldwide in the year 2019 as provided by FAO (Food and Agriculture Organization) data. Its regional production in 2019 is presented in Figure 2.
Although the main chemical components of tobacco leaves such as nicotine, protein or sugars play a significant role in tobacco quality tobacco can also have a larger quantity of heavy metals when it is cultivated in a polluted environment [58]. It can accumulate many different heavy metals, and it accumulates Cd with energy and transpiration as the driving force of its accumulation [17]. Through xylem, dissolved nutrients are translocated in all parts of the plant, where this movement is coupled with Cd translocation in Nicotiana tabacum known to promote Cd uptake and mobility as well as accumulation in leaves [59]. To emphasize that tobacco is a hyperaccumulator of Cd, implies its ability to accumulate a large amount of Cd from the soil [60]. This was observed in the pot experiment with moderately contaminated soil, where tobacco plants accumulated high concentrations of Cd in the shoots [61]. The discussion about tobacco and its metal content is very significant, as the metal toxicity continues even in tobacco products like cigarettes. For instance, investigation of toxic metals in the tobacco of different Iranian cigarettes, the study showed the presence of a higher level of heavy metals in cigarettes [11]. The consideration of the higher consumption of tobacco products and the negative effects of Cd, previous studies were interested in the determination of Cd concentration in tobacco. In the evaluation of Cd accumulation and distribution in Nicotiana species, a significantly higher level of Cd (above 80%) was accumulated in leaves of Nicotiana tabacum [62]. In the Phytoremediation potential of Atriplex canescens (Pursh) Nutt and Nicotiana tabacum has grown in heavy metal contaminated soil, tobacco plants have higher metal concentrations, transfer factor, uptake and metal phytoextraction efficiency; however, the author did not specify the accumulation potential of different parts of the plant [63]. Another study about the comparison of heavy metals accumulation and cadmium phytoextraction rate among tobacco cultivars, the quantity of accumulated Cd was higher in tobacco grown on soil with higher cadmium content [64]. In addition, Cd content was the highest in leaves which is highly attributed to higher Cd transportation from stem to leaves as it was indicated by a greater secondary transfer factor obtained in all tobacco plants in the experiment [64]. The emphasis on the accumulation of Cd in tobacco is presented in Table 1. It shows the concentration of Cd in different parts of tobacco and their corresponding soil Cd concentration that has been determined in the past studies where the findings show that the values of Cd concentration in tobacco parts were higher than the values of soil Cd in those studies [65-67].
| Mean value of Soil Cd concentration (mg/kg) | Soil pH | Plant Name | Tobacco parts | Mean Cd concentration (mg/kg) | Reference |
|---|---|---|---|---|---|
| 0.21 | 7.05 | Nicotiana tabacum L./ K326 cultivar | Leaves | 1.51 | (Haiwei L, et al.) [17] |
| Stem | 0.90 | ||||
| Roots | 0.89 | ||||
| 31.4 | 7.6 | enhanced tobacco NBCu 10-8F3 | Leaves | 125.4 | (Angelova and University-Plovdiv) [67] |
| Stem | 20.1 | ||||
| Roots | 50.5 | ||||
| 0.2 | 6.99 | tobacco | Upper Leaves | 0.24 | (Proshad R, et al.) [79] |
| Upper stem | 0.12 | ||||
| Roots | 0.17 | ||||
| 2.94 | 6.2 | tobacco | Upper leaves | 9.68 | |
| Upper stem | 4.21 | ||||
| Roots | 2.4 | ||||
| 0.99 | 5.3 | tobacco | Upper leaves | 2.66 | |
| Upper stem | 1.19 | ||||
| Roots | 0.71 | ||||
| 0.3 | 7.2 | tobacco | Upper leaves | 0.61 | |
| Upper stem | 0.35 | ||||
| Roots | 0.33 | ||||
| 0.95 | 4.5 | Burley tobacco | Leaves | 2.7 | (Golia EE, et al.) [13] |
| 0.72 | 4.7 | Virginia tobacco | Leaves | 2.5 | |
| 0.1 | 5.3 | Oriental tobacco | Leaves | 1.6 | |
| 0.21 | 7.1 | Nicotiana tabacum/ cultivar K326 | Leaves | 3.35 | (Haiwei L, et al.) [64] |
| 0.21 | 7.1 | Nicotiana tabacum/ cultivar NC89 | Leaves | 2.57 | |
| 0.4 | 5.3 | Nicotiana tabacum/ cultivar PHD6 | Leaves | 6.8 | (Michel M, et al.) [62] |
| Stem | 1.2 | ||||
| Roots | 1.3 | ||||
| 0.4 | 5.3 | Nicotiana rustica/cultivar Brasilia | Leaves | 4.6 | |
| Stem | 1.4 | ||||
| Roots | 1.8 | ||||
| 0.35 | 6.01 | Cultivar yunyan 87 | Upper leaves | 15.35 | (Qiulong H, et al.) [56] |
| Stem | 8.01 |
Table 1: Summary of concentrations of cadmium in different soils and tobacco parts from previous studies.
On the other hand, some technologies were tried to identify the way that can alleviate Cd toxicity in soil. The study was conducted to investigate how Cd contamination of soil and crops is affected by intercropping and rotation systems in the lower reaches of the Minjiang River in south-western China, which is a significantly valuable study as some plants can highly accumulate cadmium to minimize its level in the soil [66]. Other findings from another study suggest that tobacco after being cut twice still appears to accumulate high Cd in leaves in moderately contaminated acidic soils, which is evidence of how this plant can accumulate Cd from contaminated soil [67]. Moreover, the high concentration of Cd in the leaves and the high translocation factor indicate the possibility of enhanced tobacco to be used in phytoextraction [68].
Tobacco is a potential plant that promotes cadmium phytoaccumulation due to its physiology. Primary transport in the xylem, translocation in the phloem, and transport from xylem to phloem are the most important processes in the distribution of Cd in plants [69]. The tobacco plant takes advantage of the presence of appropriate genes to translocate Cd from roots to shoots and accumulate it in different parts of the plant [18]. In addition, the translocated Cd is more accumulated in tobacco leaves, and past findings confirm the presence of a higher level of Cd and other metals in tobacco and cigarettes from different countries [11]. Therefore, the movement of Cd from soil to the upper shoot follows two steps. The first step is to uptake Cd from soil to roots, which is driven by this readily available metal concentration in soil solution and other soil physicochemical factors. The second step is the translocation of up-taken metal, which is more dependent on the physiological performance of tobacco [17]. The uptake from the medium to the xylem, to be energy-dependent and the transfer from the xylem to the leaves, to be driven mainly by transpiration.
Cadmium Toxicity in Tobacco Soil and Health Risks
Cadmium (Cd) naturally found in the environment in a low concentration is known to be a soil pollutant that affects mainly soil health. Generally, soil microorganisms are particularly susceptible to the presence of heavy metals in the soil as they affect their ecological function via adsorption, ï¬xation, complexation, dissolution, oxidation or reduction [72]. Cd exhibits a toxicological effect on soil microbes, which may lead to a decrease in their numbers and activities [41]. Even though, there is no specific demonstration of the antagonist interaction between a higher level of cadmium and soil microbes. The recent works showed that the high sensitivity of the metal effect is observed in soil bacteria than in other soil microorganisms (actinomycetes, fungi, algae and protozoa), while bacteria facilitate nutrients transformation and circulation, promote energy flow together with organic matter decomposition as well as other biochemical processes in the environment [73]. For instance, Potassium-solubilizing bacteria convert insoluble potassium in the soil into a form that plants can access [74]. Therefore, disturbance in soil microbial community may affect the nutrient status of the field soil. This nonessential element has also been reported to have an antagonist relationship with zinc, which is usually an essential element for tobacco and other plants [75].
Besides, to talk about soil microbes’ exposure to cadmium is bonded together with soil structure. In the study on bacterial community response to Cd contamination of agriculture paddy soil, it has been found that long-term cadmium results in changes in bacterial population abundance and structure with significant losses in bacterial diversity, which is supposed to affect the whole soil ecosystem [72]. As long as these microorganisms are affected by the concentration of Cd in soil, it implies that there is a change in microbe’s community that may decrease and alter decomposition of organic matter, reduced soil respiration, decreased diversity and declined activity of several soil enzymes, which may result to the change of soil structure [76]. In addition to the inactivation of enzymes, Cd and other heavy metals alter the conformational structures of nucleic acids, protein, and consequently form complexes with protein molecules that render them inactive, with an overall effect of reduction in microbial biomass because of reduced biodiversity or disturbed community structure [28,29].
Moreover, when released in the environment, Cd is easily oxidized into cadmium dioxide; its vapor can react with other atmospheric gases to form water-soluble compounds [20]. Once accumulated by plants, it enters the food chain without any known biological functions in plants [77]. According to previous studies, the combination of Cd and Zn added on growing medium resulted in DNA damage in tobacco leaves [75]. The negative effects of Cd expand to even the level of human health. Cadmium has significant adverse effects on the human body, particularly on kidneys, which accumulate about 30% of body Cd [78]. It may impair Vitamin D metabolism in the kidney, affects the cardiovascular system in several ways that result in different diseases including anemia and may result in cancer [79]. It has been reviewed and presented that Cd can affect the urinary system, reproductive system, cardiovascular system, respiratory organs and other remaining parts of the human body, which shows the significant effect of cadmium exposure observed in various systems within the human body [44]. This also agreed with Tchounwou et al. who showed that Cd affects with pulmonary and gastrointestinal irritant within the human body [9].
Conclusion
Cadmium toxicity, uptake and accumulation by the tobacco plant have becoming an interesting topic because it is readily available and highly accumulated in tobacco leaves. This is happening while recent records show an increase in annual tobacco products worldwide. However, soil Cd is likely to be high in areas dominated by industries, mining, irrigation and fertilization as well as other anthropogenic activities that involve the use of products containing metals. Some soil physicochemical properties together with plant physiological properties are key determinants of the metal availability and absorption by tobacco plants. The soil pH is a primary factor that influences Cd availability and absorption by plants. Even though Cd can be up taken by various plant species; it is highly accumulated in tobacco plants. The above accumulation has been observed in many leaves of Nicotiana tabacum due to the presence of appropriate genes that promote this metal translocation from roots to shoots. Therefore, the tobacco plant is a potential accumulator of Cd originated from soil ecosystem and has the potentiality to pollute soil and create several health hazards due to the consumption of Cd contaminated tobacco leaves as tobacco products. Therefore, this current study suggests that the estimation of the efficiency of the tobacco plants in phytoremediation of Cd polluted soil may be the focus of future studies. Again, there is a need to enhance policies that promote the minimization of Cd release in the environment.
Acknowledgement
The authors would like to thank the University of Chinese Academy of Sciences, China.
References
- Andrea MME, Carolina TEA, José CBT, Luis MNJ, Carlos GML. Evaluation of contaminants in agricultural soils in an irrigation district in Colombia. Heliyon. 2019, 5(8):e02217.
- Sahadat H, Latifa GA, Prianqa, Nayeem AA. Review of cadmium pollution in Bangladesh. J Health Pollut. 2019, 9(23):190913.
- Johannes G, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, et al. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol. 2006.
- Nylund E. Cadmium uptake in willow (Salix Viminalis L.) and spring wheat (Triticum aestivum L.) in relation to plant growth and Cd concentration in soil solution. 2005.
- Kirkham MB. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma. 2006, 137(1):19-32.
- Kui C, Yu Y, Zhang M, Kim K. Concentration, source, and total health risks of cadmium in multiple media in densely populated areas, China. Int J Environ Res Public Health. 2019, 16(13):2269.
- Khan M, Khan S, Khan A, Alam M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ. 2017, 601:1591-1605.
- Ping CY, Liu Q, Liu YJ, Jia FA, He XH. Responses of soil microbial activity to cadmium pollution and elevated CO2. Sci Rep. 2014, 4(1):4287.
- Paul BT, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metals toxicity and the environment. 2012, EXS. 101:133-164.
- WHO. Preventing disease through healthy environments: Exposure to cadmium: A major public health concern. Geneva: World Health Organization. WHO IRIS. 2019.
- Catherine K, Marchetti M, Rossi L, Lugon-Moulin N. Reduction of cadmium availability to tobacco (Nicotiana tabacum) plants using soil amendments in low cadmium-contaminated agricultural soils: A pot experiment. Plant Soil. 2005, 276(1):69-84.
- Alireza P, Pourkhabbaz H. Investigation of Toxic metals in the tobacco of different Iranian cigarette brands and related health issues. Iran J Basic Med Sci. 2012, 15(1):636-644.
- Golia EE, Dimirkou A, Mitsios IK. Heavy-metal concentration in tobacco leaves in relation to their available soil fractions. Commun Soil Sci Plant Anal. 2009, 40(1-6):106-120.
- Innocent N, Osibanjo O, Oji-Nnorom CG. Cadmium determination in cigarettes available in Nigeria. African J Biotechnol. 2005.
- Huma A, Yaqub A, Malik SA, Junaid M, Yasmeen S, Abdullah MA. Characterization of toxic metals in tobacco, tobacco smoke, and cigarette ash from selected imported and local brands in Pakistan. Sci World J. 2014.
- Ishaq E. Analysis of heavy metals in selected cigarettes and tobacco leaves in benue state, Nigeria. Ohio J Sci. 2013, 3:244-247.
- Haiwei L, Wang H, Ma Y, Wang H, Shi Y. Role of Transpiration and metabolism in translocation and accumulation of cadmium in tobacco plants (Nicotiana tabacum L.). Chemosphere. 2016, 144:1960-1965.
- Victor H, Julio E, Borne FD, Punshon T, Ricachenevsky FK, et al. Inactivation of Two newly identified tobacco heavy metal ATPases leads to reduced Zn and Cd Accumulation in shoots and reduced pollen germination. Metallomics. 2014, 6(8):1427-1440.
- Yasir H, Tang L, Wang X, Hussain B, Yaseen M, et al. Immobilization of cadmium and lead in contaminated paddy field using inorganic and organic additives. Sci Rep. 2018, 8(1):17839.
- WHO. Inorganic pollutants: Cadmium. In Air Quality Guidelines for Europe. 2nd Edition, Denmark. 2000.
- Baker DE, Amacher MC, Leach RM. Sewage sludge as a source of cadmium in soil-plant-animal systems. Environ Health Perspect. 1979, 28:45-49.
- Chang P, El-Amamy M. Cadmium levels in soils and crops in the United States. In Lead, Mercury, Cadmium and Arsenic in the Environment. John Wiley & Sons Ltd. 1987.
- Riley RG, Zachara JM. Chemical contaminants on doe lands and selection of contaminant mixtures for subsurface science research. Pacific Northwest Lab., Richland, WA (United States). 1992.
- Xiangyang B, Feng X, Yang Y, Qiu G, Li G, et al. Environmental contamination of heavy metals from zinc smelting areas in hezhang county, western guizhou, China. Environ Int. 2006, 32(7):883-890.
- Yasir H, Tang L, Sohail M, Cao X, Hussain B, et al. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci Total Environ. 2019.
- Bambang H, Winarso S, Kusumandaru W. soil chemical properties index of tobacco plantation land in Jember District. Agric Sci Procedia. 2016.
- Aneta Z, Sarzynska M, Szpyrka E, Stawarczyk K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230(7):164.
- Ademola OO, Balgobind A, Pillay B. Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci. 2013, 14(5):10197-10228.
- Anna LB, Boron P. The effect of industrial heavy metal pollution on microbial abundance and diversity in soils-a review. Environ Risk Assess Soil Contam. 2014.
- Radka B. Heavy metal concentrations in soil and tobacco plants following long-term phosphorus fertilization. Bulg J Agric Sci. 2016, 22:16-20.
- Fatemeh S, Ghasemi S, Sodaiezadeh H, Ayaseh K, Zamani-Ahmadmahmoodi R. The effect of sewage sludge on heavy metal concentrations in wheat plant (Triticum aestivum L.). Environ Sci Pollut Res Int. 2017.
- Raymond W, Okieimen F. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011.
- Chao L, Lu L, Huang T, Huang Y, Ding L, et al. The distribution and health risk assessment of metals in soils in the vicinity of industrial sites in Dongguan, China. Int J Environ Res Public Health. 2016, 13(8).
- Hutton M. Sources of cadmium in the environment. Ecotoxicol Environ Saf. 1983, 7(1):9-24.
- Hubert H, Nshimiyimana FX, Ngendahayo E, Akimpaye B, Nahayo L, et al. Evaluation of soil contamination in mining areas of Rwanda. 2019.
- Åsgeir RA, Manoko MLK. Trace element concentrations in soil, sediments, and waters in the vicinity of geita gold mines and north mara gold mines in northwest Tanzania. Soil Sediment Contam Int J. 2012, 21(2):135-159.
- Manfred FB, Kweyunga C, Manoko MLK. Levels of heavy metals and cyanide in soil, sediment and water from the vicinity of north mara gold mine in Tarime district, Tanzania. Tanzania: Christian Council of Tanzania (CCT). 2009.
- Oluwatosin O, Olayinka A, Awotoye O. Ecological impact of mining on soils of South-western Nigeria.” Environ Exp Biol. 2014, 12:179-186.
- Dorleku MK, Nukpezah D, Carboo D. Effects of small-scale gold mining on heavy metal levels in groundwater in the lower Pra Basin of Ghana. Appl Water Sci. 2018, 8(5):126.
- Haiwei L, Wang H, Zhang Y, Wang H, Yang J, et al. Comparison of heavy metal accumulation and cadmium phytoextraction rates among ten leading tobacco (Nicotiana tabacum L.) cultivars in China.” Int J Phytoremediation. 2019, 21(7):699-706.
- Khan S, Hesham AEL, Qiao M, Rehman S, He JZ. Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut Res Int. 2010, 17(2):288-296.
- DalCorso G, Silvia F, Antonella F. Regulatory networks of cadmium stress in plants. Plant Signal Behav. 2010, 5(6):663-667.
- Girish C, Saifullah, Bolan N, Bibi S, Iqbal M, et al. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci. 2014, 33(5):374-391.
- Mehrdad RR, Rahimzadeh MR, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: An update. Caspian J Intern Med. 2017, 8(3):135-145.
- Yu S, Jin L, Wang X. Cadmium absorption and transportation pathways in plants. Int J Phytoremediation. 2017, 19(2):133-141.
- Ogundiran MB, Osibanjo O. Mobility and speciation of heavy metals in soils impacted by hazardous waste. Chem Speciation Bioavailability. 2009, 21(2):59-69.
- Ali S, Soylak M, Ozcan H. Investigation of heavy metal mobility and availability by the BCR sequential extraction procedure: Relationship between soil properties and heavy metals availability. Chem Speciation Bioavailability. 2014, 26(4):219-230.
- Domergu FL, Védy JC. Mobility of heavy metals in soil profiles. Int J Environ Anal Chem. 1992, 46(1-3):13-23.
- Rieuwerts JS, Thornton I, Farago ME, Ashmore MR. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem Speciation Bioavailability. 1998, 10(2):61-75.
- Autumn SW, Angle JS, Chaney RL, Delorme TA, Reeves RD. Soil PH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil. 2006, 281(1):325-337.
- Huan-Yun Y, Liu C, Zhu J, Li F, Deng DM, et al. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and PH value. Environ Pollut. 2015, 209:38-45.
- Aaron S, Jeyakumar P, Hedley M, Anderson C. Influence of soil moisture status on soil cadmium phytoavailability and accumulation in plantain (Plantago lanceolata). Soil Systems. 2018, 2:1-15.
- Ling, Li Y, Liu JT, Hu L, Chen X. Plant coexistence can enhance phytoextraction of cadmium by tobacco (Nicotiana tabacum L.) in contaminated soil. J Environ Sci. 2011, 23(3):453-460.
- Qiyu D, Fang J, Huang F, Cai K. Silicon amendment reduces soil Cd availability and Cd uptake of two pennisetum species. IntJ Environ Res Public Health. 2019, 16(9).
- Qun R, Zhong K, Huang H, Li C, Zhang C, et al. Humic acid reduces the available cadmium, copper, lead, and zinc in soil and their uptake by tobacco. 2020.
- Qiulong H, Zeng W, Li F, Huang Y, Gu S, et al. Effect of nano zeolite on the transformation of cadmium speciation and its uptake by tobacco in cadmium-contaminated soil. Open Chem. 2018, 16:667-673.
- Teh-wei H, Lee AH. Tobacco control and tobacco farming in African countries. J Public Health Policy. 2015, 36:41-51.
- Wang M, Duan S, Zhou Z, Chen S. Alleviation of cadmium toxicity to tobacco (Nicotiana tabacum) by biofertilizers involves the changes of soil aggregates and bacterial communities. Ecotoxicol Environ Saf. 2018, 169:240-247.
- George JW, Yeargan R. Variation in cadmium accumulation potential and tissue distribution of cadmium in tobacco. Plant Physiol. 1986, 82(1):274-279.
- Charles C, Rini DS. Cadmium contamination and the role of bioaccumulator plant as a remediation agent. AIP Conference Proceedings 2014. 2018, 1: 020126.
- Zhong T, Cai H, Li J, Lv Y, Zhang W, et al. allelic variation of NtNramp5 associated with cultivar variation in cadmium accumulation in tobacco. Plant Cell Physiol. 2017, 58(9):1583-1593.
- Michel M, Tancogne J, Gomez A, Juste C. Cadmium bioavailability to Nicotiana tabacum L., Nicotonia Rustica L., and Zea mays L. grown in soil amended or not amended with cadmium. Biol Fertility Soil. 1989, 8(1):48-53.
- Topcuoglu B, Nuri A, Houcine ARY. Phytoremediation potential of Atriplex canescens (Pursh) nutt and Nicotiana tabacum grown in heavy metal contaminated soil. International Conference on Food, Nutrition and Agriculture, Turkey. 2019.
- Haiwei L, Wang H, Zhang Y, Wang H, Yang J, et al. Comparison of Heavy metal accumulation and cadmium phytoextraction rates among ten leading tobacco (Nicotiana tabacum L.) cultivars in China.” Int J Phytoremediation. 2019, 21(7):699-706.
- Proshad R, Dan Z, Minhaz U, Yi W. Presence of cadmium and lead in tobacco and soil with ecological and human health risks in Sichuan province, China. Environ Sci Pollut Res. 2020, 27(15):18355-18370.
- Junhong B, Xiao R, Cui B, Zhang K, Wang Q, et al. Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the pearl river estuary, South China. Environ Pollut. 2011, 159(3):817-824.
- Yang Y, Ge Y, Zeng H, Zhou X, Peng L, et al. Phytoextraction of cadmium-contaminated soil and potential of regenerated tobacco biomass for recovery of cadmium. Sci Rep. 2017.
- Angelova V, Mendeleev Street Plovdiv Bulgaria Agricultural University-Plovdiv. Phytoremediation potential of enhanced tobacco in soil contaminated with heavy metals. 2018.
- Girish C, Saifullah, Bolan N, Bibi S, Iqbal M, et al. Cellular Mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci. 2014, 33(5):374-391.
- Vögeli-Lange R, Wagner GJ. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves: Implication of a transport functions for cadmium-binding peptides. Plant Physiol. 1990, 92(4):1086-1093.
- Jie Z, Martinoia E, Lee Y. Vacuolar transporters for cadmium and arsenic in plants and their applications in phytoremediation and crop development. Plant Cell Physiol. 2018, 59(7):1317-1325.
- Luo LY, Xie LL, Jin DC, Mi BB, Wang DH, et al. Bacterial Community response to cadmium contamination of agricultural paddy soil. Appl Soil Ecol. 2019, 139:100-106.
- He Z, Li X, Zhang Z, Zhao Y, Chen P, et al. Drivers and assemblies of soil eukaryotic microbes among different soil habitat types in a semi-arid mountain in China. Peer J. 2018, 6:e6042-e6042.
- Chengsheng Z, Kong F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol. 2014, 82:18-25.
- Mirta T, Štefanic PP, Cvjetko P, Šikic S, Pavlica M, et al. The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants. PLOS ONE. 2014, 9(1):e87582.
- Weilin S, Ma X. Effects of heavy metal cd pollution on microbial activities in soil. Ann Agric Environ Med. 2017, 24(4):722-725.
- Voijant TB, Abdullah SRS, Basri H, Idris M, Anuar N, et al. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. 2011, 939161.
- Robin AB. Cadmium toxicity and treatment. Sci World J. 2013, 394652.
- Soisungwan S, Garrett SH, Sens MA, Sens DA. Cadmium, Environmental exposure, and health outcomes. Environ Health Perspect. 2010, 118(2):182-190.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences