ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
A Curcumin Phospholipid Complex Formulation Induces Neurogenesis and Prevents Anoikis-Induced Cell Death in an In Vitro Gut-Brain Axis Model
Marcel B1, Dana L2, Raphael S1 and Yehoshua M1*
1 Phytor Lab for Drug Development, Hadassah Medical Center, Hebrew University Biotechnology Park, Jerusalem, Israel
2 Bara Herbs Ltd. Ha-Atid Road 1, Yoqneam, Israel.
- *Corresponding Author:
- Yehoshua M
Phytor Lab for Drug Development, Hadassah Medical Center
Hebrew University, Biotechnology Park, Jerusalem, Israel
Tel: 97225025229
E-mail: yehoshua.maor@mail.huji.ac.il
Received Date: July 04, 2018; Accepted Date: July 09, 2018; Published Date: Juy 11, 2018
Citation: Yehoshua M, Marcel B, Dana L, et al. (2018) A Curcumin Phospholipid Complex Formulation Induces Neurogenesis and Prevents Anoikis-Induced Cell Death in an in vitro Gut-Brain Axis Model. Am J Phytomed Clin Ther Vol.6 No.2:10 DOI: 10.21767/2321-2748.100346
Abstract
Curcumin, a plant polyphenol with potent anti-inflammatory, anti-oxidant, and neuroprotective properties, continues to attract interest in the dietary supplement industry as well as in natural products research. However, its low solubility and poor bioavailability as a natural ingredient hinders its optimal preventive and therapeutic outcomes. The current study delves into the mechanisms of action of a phospholipid-based formulation; namely, Bara Curcumin Effect (BCE), which combines phospholipids from soy lecithin with curcumin to enhance its bioavailability. By measuring the fluorescence of curcumin in its specific wavelength (Ex/Em=485/530 nm), we detected it in the cytoplasm after treatment with BCE. Additional experiments, including anoikis-induced cell death, IL-1β- induced toxicity, and western blots analysis, corroborated the protective effect of BCE and its subsequent stimulation of cell survival. BCE also significantly induced neurogenesis on SH-SY5Y cells. These in vitro results demonstrated that BCE possesses significant anti-inflammatory and protective effects. BCE was also capable of inducing neurogenesis, which invites further studies on the mechanism of action of BCE since it may reduce chronic inflammation and rescue damaged tissue. Our studies align with previous studies exhibiting the association between chronic gastrointestinal inflammation and increased risk of neurological diseases.
Keywords
Curcumin phospholipid complex; Neurogenesis; Anoikis; Gut-brain axis; in vitro
Introduction
Regardless of the growing knowledge in neurological research, the incidence of neuroinflammatory diseases including Parkinson and Alzheimer’s has achieved alarming levels in the elderly population and is growing significantly every year [1]. Unfortunately, apart from the scientific knowledge that has further disclosed the mechanism underlying the ontogenesis of those diseases, there is currently no available treatment leading to a cure [2]. However, recent promising research has demonstrated that gut health status is pivotal in determining the initiation and the progression of neurological diseases and not only the brain [3,4]. In fact, the gut is the central barrier that when compromised may imply early biochemical disturbances preceding the onset of any clinical symptom or any measurable or detectable biological alteration. In the past ten years, extensive research revealed a number of phytochemicals, polyphenols, and polyunsaturated fatty acids (e.g., resveratrol, quercetin, coumaric acid, omega-3 from linseed oil, and gamma-linolenic acid from borage oil) with potent anti-inflammatory effects, heralding a new era in the quest for potential candidates for neurological disease therapies [5]. Curcumin has already been used to treat several inflammatory disorders including diabetes, obesity, cardiovascular and neurodegenerative diseases, cerebral edema, allergy, arthritis, inflammatory bowel disease (IBD), renal ischemia, scleroderma, psoriasis, and various cancers [6]. Other conditions include anorexia, hepatic disorders, biliary disorders, sinusitis, wound healing, and rheumatism [7,8]. Curcumin`s potential effects also include antibacterial [9], antioxidant, hypoglycemic, anti-angiogenic, and apoptotic activities [10]. The multitarget effect of curcumin, which is clinically relevant for the general improvement of the individual’s health, is now known to be mediated by interaction with multiple cell signaling molecules including proinflammatory cytokines, apoptotic proteins, transcription factors, various enzymes, and proteins. It is well established that Curcumin inhibits NF-kB – regulated inflammatory genes. It also binds to and inhibits Cyclooxygenase 2 (COX-2), 5-Lipoxygenase (5-LOX), and IkB-α kinase enzymatic activity. The inhibition of inflammatory genes activated by NF-kB avoids the occurrence of chronic illnesses [11,12]. The Food and Drug Administration (FDA) has approved curcumin as a dietary nutrient, which is safe and well tolerated. Curcumin is used in capsules, tablets, energy beverages, and cosmetics [13] however, its poor bioavailability creates a very challenging situation. Therefore, extensive research aims to create new formulas and carriers able to enhance curcumin`s absorption in the cells. The incorporation of curcumin on phospholipid micelles is a frequently used strategy to improve its bioavailability since it is a very lipophilic compound. This study reveals essential in vitro data, comparing the efficiency of the curcumin-phospholipid complex, named Bara Curcumin Effect (BCE) with pure curcumin.
Materials and Methods
Cell culture
Adherent human colon cancer Caco-2 and neuroblastoma SH-SY5Y cell lines were purchased from the American Type Culture Collection (ATCC), being cultured according to standard mammalian tissue culture protocols and sterile conditions. SHSY5Y were cultured in RPMI 1640 medium and Caco-2 in high glucose Dulbecco’s Modified Eagle Medium (DMEM). All media were supplemented with 10% fetal bovine serum, streptomycin (100 μg/ml), penicillin (100 U/ml), and Nystatin (12.5 U/ml). Cells were incubated in a 5% CO2 containing atmosphere at 37°C. All tissue cultures were maintained in 25 cm2 NuncTM cell culture treated with EasYFlaskTM (Thermo Fisher Scientific), and all the media and supplements were obtained from Biological Industries. The Trypan Blue exclusion method was used for plating viable cells as well as for counting them after treatment.
MTT assay
The cell viability following the treatment was determined using a commercially available MTT assay kit (ABCAM, ab146345) and performed according to manufacturer's instructions. Briefly, cells were seeded in a 96-well plate at a density of 1 × 104 cells/well (n=4). After overnight stabilization, cells were exposed to varying concentrations of curcumin and BCE. Plates were incubated in a humidified atmosphere containing 5% CO2 in air at 37°C for 72 hours. According to the MTT standard protocol, after the treatment period, the media was removed, and all cells were incubated with serum-free media containing 0.5 mg/ml MTT for three to four hours in the incubator. The MTT purple crystals formed by the viable cells were diluted using isopropanol containing 0.04 mol/L HCl. The quantification was determined by measuring the optical density at 570 nm in an enzyme-linked immunosorbent assay (Spark, Tecan) reader. Data were presented as proportional viability (%) by comparing the treated group with the untreated cells (i.e., treated with the vehicle only), the viability of which is assumed to be 100%.
Transepithelial electrical resistance (TEER)
Caco-2 colon cell lines were plated in a 6.5 mm Transwell with 0.4 μm pore polycarbonate membrane insert (Millipore). SH-SY5Y cells were plated in the basolateral side on the bottom of the well in a 24-well polystyrene plate. Seventy-two hours after seeding, treatment was set for 24 hours. TEER measurement was used to determine the monolayer integrity. At the end of the procedure, plates were equilibrated at room temperature for one hour. The electrical resistance (ER) across the monolayer was measured using an ohmmeter (Millicell) equipped with probes, with one probe positioned inside the filter well and the second into the medium in the growth well. The ER of a blank insert (without cells) was determined to subtract the background value.
Imaging and fluorescent analysis
For fluorescent analysis on the microplate reader: after a onehour treatment the cells growing in a 96-well plate were washed with PBS to remove external curcumin and digested with a RIPA buffer for lysis to release curcumin from inside the cells. The reading was then performed using curcumin`s specific wavelength (Ex/Em=485/530 nm). For imaging analysis: after one hour of treatment, the fixation of cells growing in a 24-well plate was performed using 0.4% paraformaldehyde for twenty minutes. The cellular nucleus was stained using 5 μg/ml Hoechst 33342. The analysis was performed using a Nikon-TL fluorescent microscope from The Hebrew University of Jerusalem Interdepartmental Equipment Facility.
Immunoblot analysis
Immunoblot analysis was performed using antibodies against KRAS (1:2000 dilution; ab157255, Abcam), p65 (1:2000 dilution; ab16502, Abcam), E- cadherin (1:10000 dilution; ab40772, Abcam), and the internal control GAPDH (1:6000 dilution; ab128915, Abcam). Species-specific HRP-labeled secondary antibodies were used. The blots were visualized using enhanced chemiluminescence (biological industries) and quantitated using Image Quant LAS 4000 mini, General Electric).
HPLC analysis: HPLC analysis was carried out to normalize the concentration of curcumin used in the biological assays. Thirty μg/ ml aliquots of curcumin and BCE were collected using preparative HPLC (Dionex UltiMate 3000, Thermo Scientific). Each mg/ml Curcumin 95% is relative to 2.8 mg/ml of the curcumin molecule from BCE. HPLC was carried out using Acclaim C18 column (2.1 × 150 mm, particle size 2.2 μm, Dionex) and Luna C18(2) column (Phenomenex, 4.6 × 250 mm, particle size 5 μm).
Statistical analysis: We used Student's t-test for statistical analysis in Microsoft Excel. Data were considered significantly different from control when p<0.05.
Results and Discussion
Measuring/imaging curcumin inside the cells
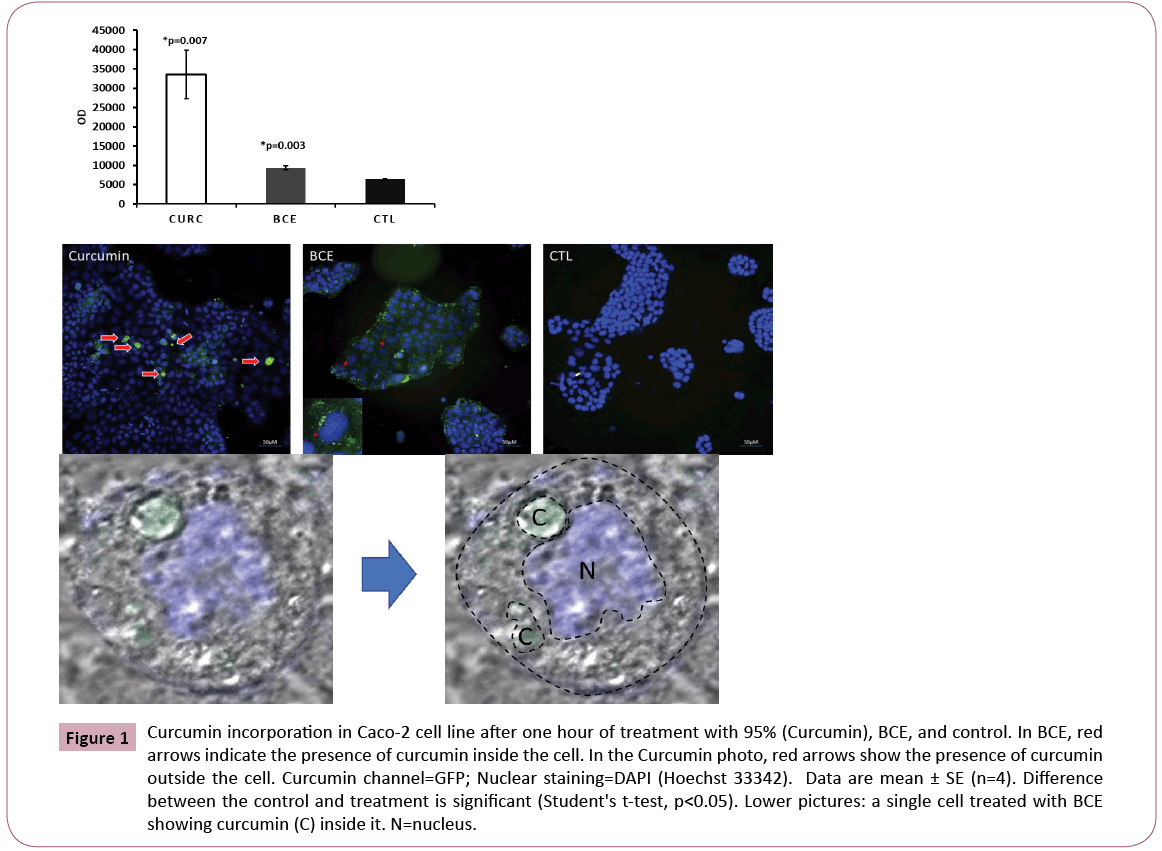
Research has demonstrated that the plasma concentrations of curcumin in patients taking relatively high oral doses of this compound are very low, typically in the nanomolar range [14]. Curcumin undergoes extensive metabolism in the intestine and liver [15,16]. In clinical trials with patients who have pancreatic cancer, circulating curcumin was detectable only as the glucuronide and sulfate conjugate forms and not as curcumin anymore, albeit at low steady-state levels, which demonstrates its poor oral bioavailability [6]. In this study we aimed at checking the efficiency of BCE in delivering curcumin inside the cells in comparison with free curcumin (Curcumin 95%) using the Caco-2 cell line model. The results confirmed the delivery and the presence of curcumin inside the cells, but not more than the Curcumin 95% itself. This puzzling effect was further elucidated by imaging analysis (Figure 1) which demonstrated a significant amount of curcumin from the Curcumin 95% samples outside the cells. Curcumin remains attached to the plasma membrane or even in the plate outside the cells, which is made of polypropylene material, known to have a strong affinity to the curcumin molecule [17]. Thus, BCE delivered curcumin molecules more efficiently to the intracellular space compared with Curcumin 95%. These observations could be relevant for clinical management of the dose, avoiding the need for taking excessive amounts of curcumin, which may cause severe adverse gastrointestinal events including nausea and diarrhea. It also may cause an increase in serum alkaline phosphatase and lactate dehydrogenase in doses ranging from 0.9 to 3.6 g/day for one to four months [18,19].
Figure 1: Curcumin incorporation in Caco-2 cell line after one hour of treatment with 95% (Curcumin), BCE, and control. In BCE, red arrows indicate the presence of curcumin inside the cell. In the Curcumin photo, red arrows show the presence of curcumin outside the cell. Curcumin channel=GFP; Nuclear staining=DAPI (Hoechst 33342). Data are mean ± SE (n=4). Difference between the control and treatment is significant (Student's t-test, p<0.05). Lower pictures: a single cell treated with BCE showing curcumin (C) inside it. N=nucleus.
Effect of BCE on improving cellular tightness
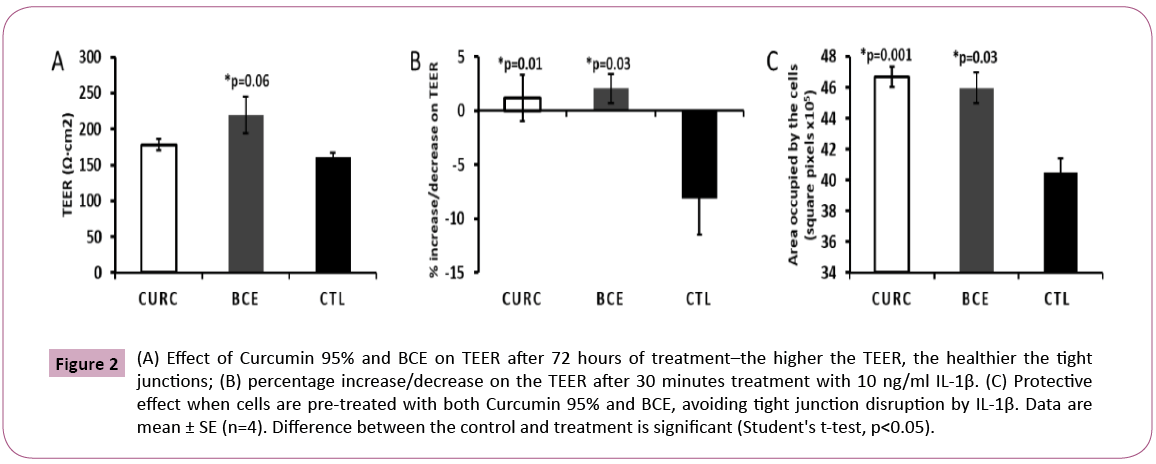
Curcumin per se increases the tightness of cellular tight junctions and inhibits the disrupting effect of IL1-β on tight junctions [20,21]. Primarily, without any induction of cellular stress, we measured the TEER after 72 hours of treatment. Surprisingly, BCE showed persistently high TEER values as opposed to Curcumin 95% and control (Figure 2). This preliminary data allowed us to conclude that BCE may be more efficient than the noncomplexed curcumin in maintaining the tight junction integrity during longer periods. This outcome encourages future analysis of BCE’s bioavailability in vivo. Furthermore, we also compared the ability of BCE in protecting cells from IL1-β-induced tight junction disruption. For that purpose, we pre-treated the cells with Curcumin 95% and BCE for thirty minutes and then exposed the cells to 10 ng/ml IL1-β for another thirty minutes. Following that, we measured the TEER and captured images in a separate assay to show the integrity of the tight junctions in the Caco-2 cell line (Figure 2). In line with the literature, pre-treatment with Curcumin 95% displayed a substantial protecting effect, eliminating the tight junction disruption induced by further incubation with interleukin. Moreover, just as for Curcumin 95%, BCE revealed significant protection, showing that both are capable of promoting that effect in vitro.
Figure 2: (A) Effect of Curcumin 95% and BCE on TEER after 72 hours of treatment–the higher the TEER, the healthier the tight junctions; (B) percentage increase/decrease on the TEER after 30 minutes treatment with 10 ng/ml IL-1β. (C) Protective effect when cells are pre-treated with both Curcumin 95% and BCE, avoiding tight junction disruption by IL-1β. Data are mean ± SE (n=4). Difference between the control and treatment is significant (Student's t-test, p<0.05).
Effect of BCE on neurogenesis in the gut-brain axis model
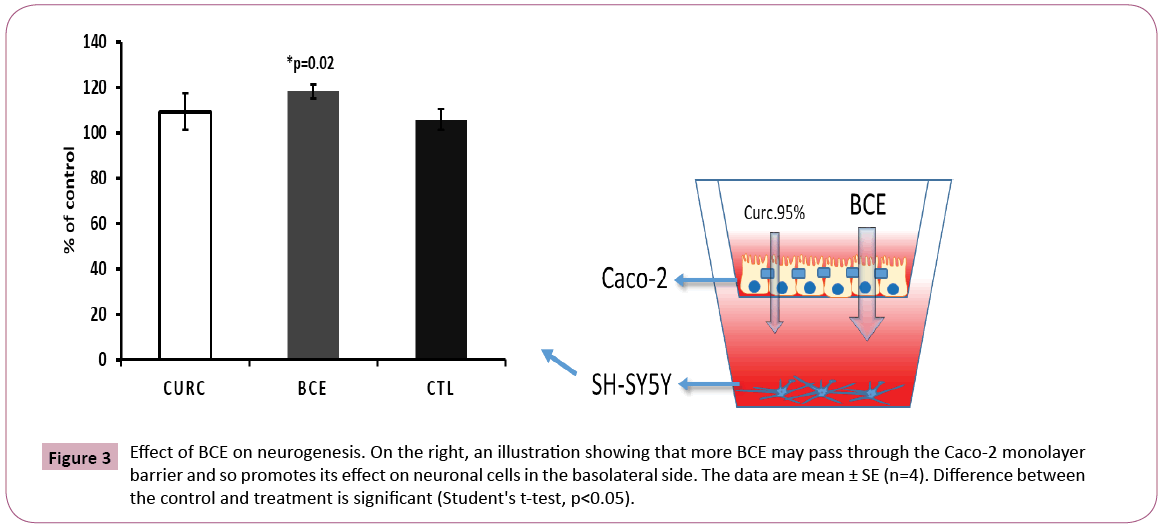
Analysis of neurons (SH-SY5Y cells), which were plated in the basolateral side on the bottom of the well, showed a significant increase in cell proliferation after BCE treatment, but not for Curcumin 95% (Figure 3). This result confirmed that BCE is more effective in passing through the Caco-2 monolayer barrier, then accessing the neuronal cells below. It is worthwhile to consider future in vivo assays using neurodegenerative disease models to explore BCE’s effect on neurogenesis.
Figure 3: Effect of BCE on neurogenesis. On the right, an illustration showing that more BCE may pass through the Caco-2 monolayer barrier and so promotes its effect on neuronal cells in the basolateral side. The data are mean ± SE (n=4). Difference between the control and treatment is significant (Student's t-test, p<0.05).
BCE prevents anoikis-induced cell death
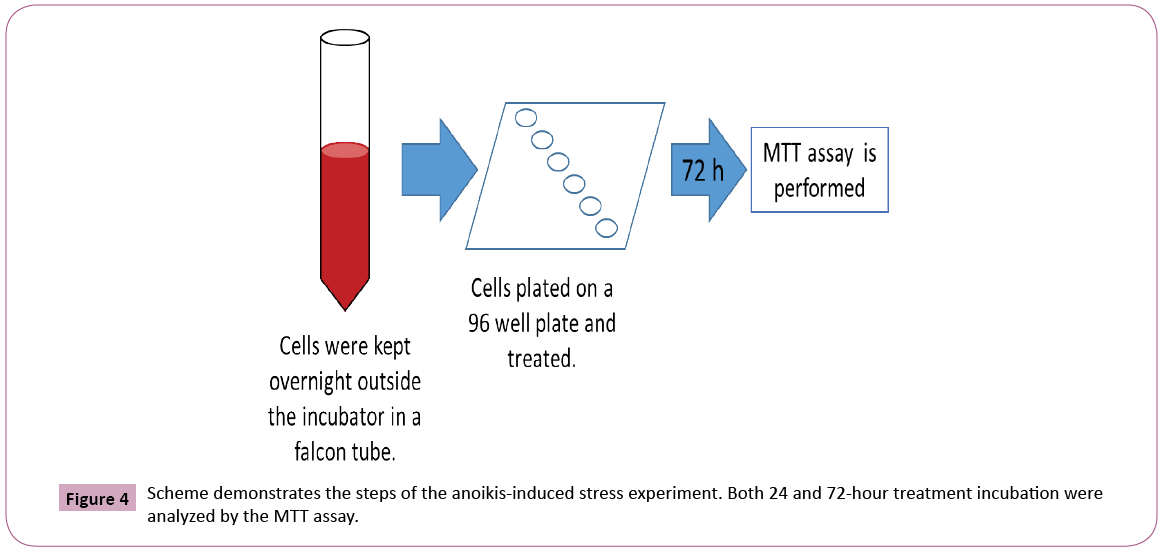
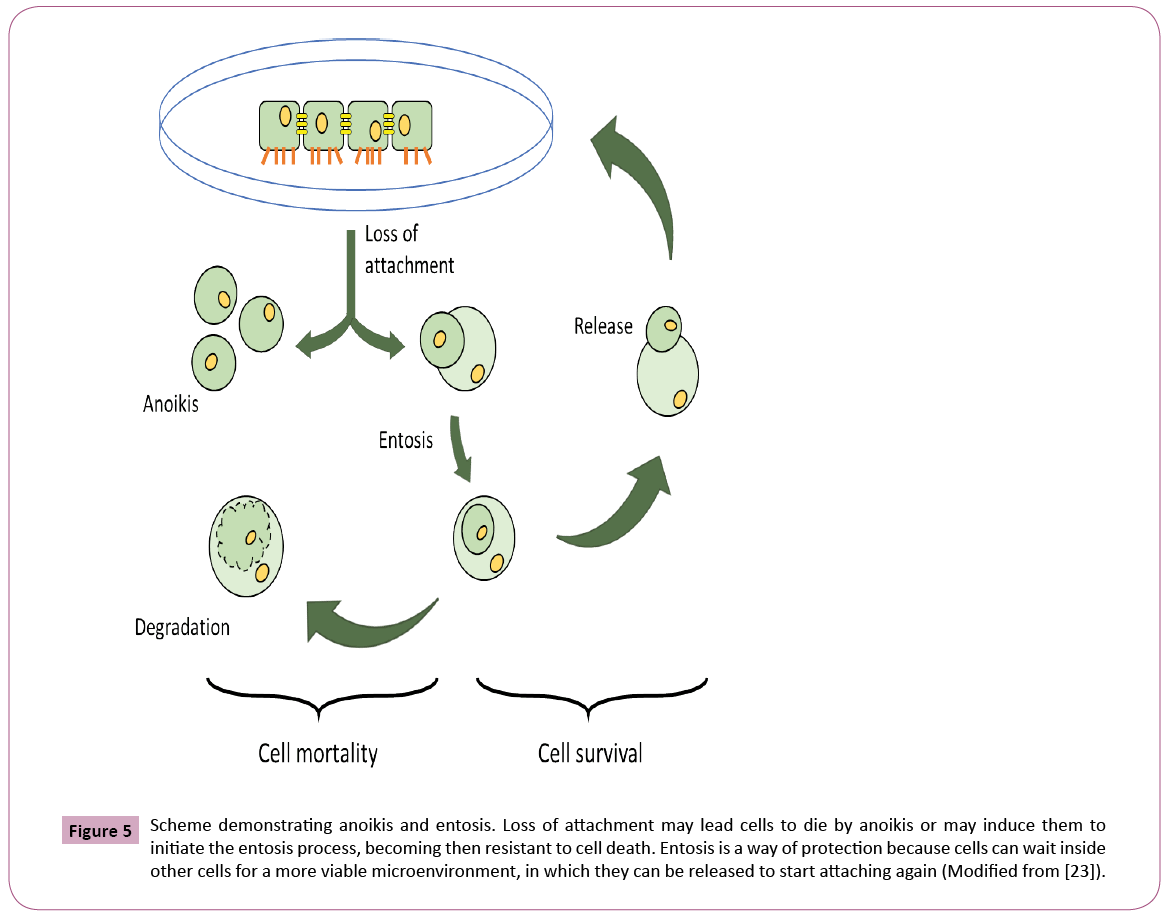
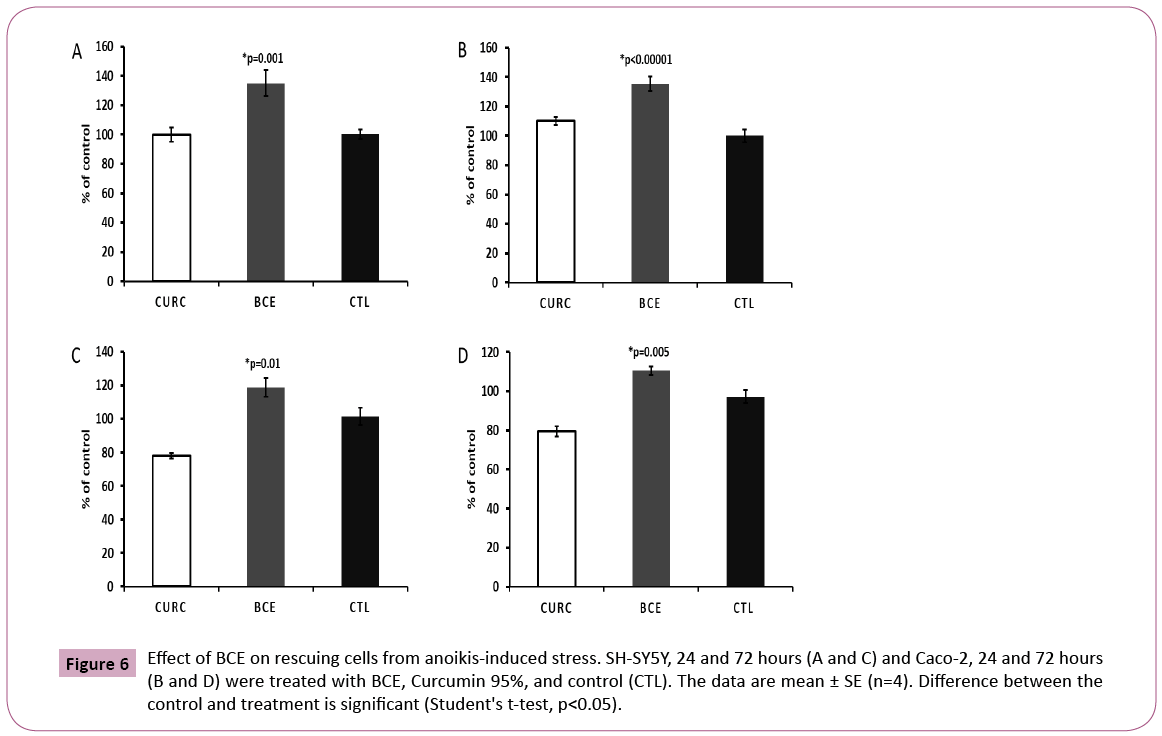
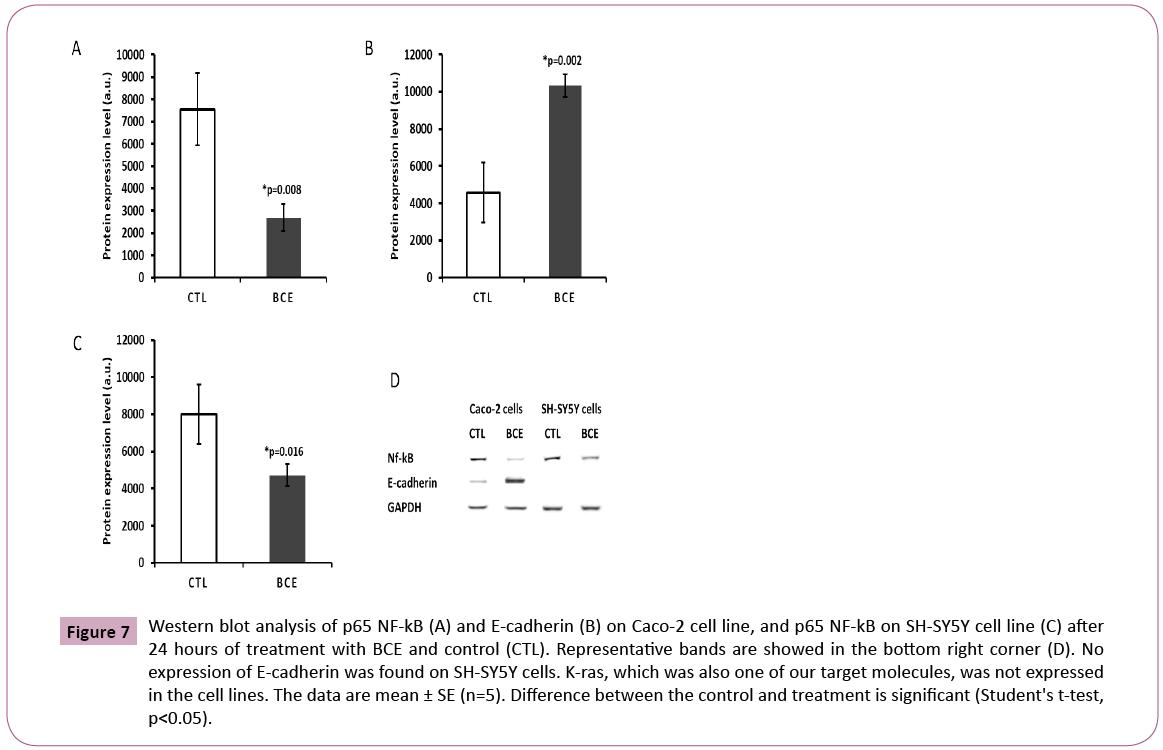
The ability of BCE in preventing anoikis-induced cell death represents the most intriguing data obtained in this study. The assay was developed in our lab and consists of keeping trypsinized cells outside the incubator (at room temperature) in a 15 ml falcon tube with the respective media (DMEM for Caco-2 and RPMI for SH-SY5Y cells) containing 10% FBS (Figure 4). In this way, cells were not able to attach, forcing them to initiate the process of cell death induced by anoikis (Figure 5), by definition, is a form of programmed cell death that occurs in anchorage-dependent cells when they detach from the surrounding extracellular matrix [22]. After overnight incubation under this stress condition, cells were placed in a 96-well plate and treated for 24 or 72 hours, followed by MTT cell viability analysis (Figure 4). The results presented here demonstrate that BCE, but not Curcumin 95%, is able to exert a protective effect on the cells, allowing a significant percentage of cells to be rescued from the pre-imposed stress conditions (Figures 5 and 6). Regarding the mechanism of action on the anoikis-induced stress, cells can initiate a tentative survival process called entosis (Figure 5), rendering the cells in a dormant state until they receive a signal from the microenvironment, thereby circumventing anoikis [23]. So, we thought that the referred signal could be increased when cells are exposed to BCE. By knowing that the loss of cell adhesion triggers metabolic and oxidative stress responses [23], it is believed that BCE could stimulate still living engulfed cells (entotic cells) to be released from the host cell to reattach and grow because of BCE’s rapid recovery from oxidative stress. That is why after 24 or 72 hours we found more viable cells on the samples treated with BCE. Moreover, western blot analysis for E-cadherin and p65 NF-kB strongly suggest the mechanisms for entosis. The up-regulation in E-cadherin protein levels is a crucial biochemical path that encourages cells to induce entosis [24]. In line with that, BCE was significantly able to increase E-cadherin expression (Figure 7). On the other hand, the p65 NF-kB protein expression, which is known to be activated by reactive oxygen species (ROS), was significantly down-regulated (Figure 7), which means that the antioxidant activity of BCE is indeed able to decrease the ROSdependent
Figure 5: Scheme demonstrating anoikis and entosis. Loss of attachment may lead cells to die by anoikis or may induce them to initiate the entosis process, becoming then resistant to cell death. Entosis is a way of protection because cells can wait inside other cells for a more viable microenvironment, in which they can be released to start attaching again (Modified from [23]).
Figure 6: Effect of BCE on rescuing cells from anoikis-induced stress. SH-SY5Y, 24 and 72 hours (A and C) and Caco-2, 24 and 72 hours (B and D) were treated with BCE, Curcumin 95%, and control (CTL). The data are mean ± SE (n=4). Difference between the control and treatment is significant (Student's t-test, p<0.05).
Figure 7: Western blot analysis of p65 NF-kB (A) and E-cadherin (B) on Caco-2 cell line, and p65 NF-kB on SH-SY5Y cell line (C) after 24 hours of treatment with BCE and control (CTL). Representative bands are showed in the bottom right corner (D). No expression of E-cadherin was found on SH-SY5Y cells. K-ras, which was also one of our target molecules, was not expressed in the cell lines. The data are mean ± SE (n=5). Difference between the control and treatment is significant (Student's t-test, p<0.05).
NF-kB activation.
Conclusions
According to our in vitro results, curcumin in a phospholipid complex demonstrated enhanced absorption, achieving the inner compartment of the cells more efficiently than Curcumin 95% (i.e., not complexed with phospholipids), which remained in significant amounts outside the cells. Anti-inflammatory and protective effects on both colon (Caco-2) and neuronal (SH-SY5Y) derived cell lines were significantly exhibited after the treatment with a curcumin-phospholipid complex. This result was confirmed by western blot analysis of the p65 NF-kB protein, which was down-regulated in both cell lines after treatment. Neurogenesis was induced in a gut-brain axis model, demonstrating to have a higher diffusion through the Caco-2 cell monolayer with curcumin-phospholipid complex as opposed to Curcumin 95%. Curcumin enhanced cell tightness in the Caco-2 cell monolayer on the apical side, which was demonstrated by the augmentation of the TEER and the elevation of E-cadherin protein expression. Curcumin-phospholipid complex inhibited the IL-1β-induced permeability of the Caco-2 cell monolayer. Also, inhibition of anoikis-induced cell death, probably by the induction of entosis, in an E-cadherin-dependent mechanism, was noticed after the treatment with the curcumin-phospholipid complex. The data presented here are in agreement with the use of curcumin herbal supplementation in the human diet and encourages the use of curcumin-phospholipid complex to increase its bioavailability in the body. Thus, it should be considered taking Curcumin- Phospholipid Complex formulations like BCE instead of curcumin alone. This may help efficiently reduce inflammatory processes in the digestive system and thus prevent neurological disorders, since there is an association between chronic gastrointestinal inflammation and increased risk of neurological diseases like Alzheimer and Parkinson’s disease.
References
- Hofman A, Jong PT, Duijn CM, Breteler MM (2006) Epidemiology of neurological diseases in elderly people: what did we learn from the rotterdam study. Lancet Neurol 5: 545-550.
- Janigro D (2006) The cell cycle in the central nervous system. Humana Press.
- Papathanasiou A, Nikakis P, Bonakis A (2014) Rapidly progressive dementia as presenting feature in Inflammatory bowel disease. Alzheimer Dis Assoc Disord 28: 294-295.
- Lin JC, Lin CS, Hsu CW, Lin CL (2016) Association between Parkinsonʼs disease and inflammatory bowel disease. Inflamm Bowel Dis 22: 1049-1055.
- Alamgir ANM (2017) Therapeutic use of medicinal plants and their extracts.
- Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15: 195-218.
- Aggarwal BB, Sung B (2009) Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 30: 85-94.
- Sharma P, Singh R (2011) Hepatoprotective effect of curcumin on lindane-induced oxidative stress in male wistar rats. Toxicol Int 18: 124-129.
- Schraufstatter E, Bernt H (1949) Antibacterial action of curcumin and related compounds. Nature 164: 456-457.
- Somparn P, Phisalaphong C, Nakornchai S, Unchern S (2007) Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull 30: 74-78.
- Menon VP, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 595: 105-125.
- Smith AJ, Oertle J, Prato D (2015) Multiple actions of curcumin including anticancer, anti-inflammatory, antimicrobial and enhancement via cyclodextrin. J Cancer Ther 6: 257-272.
- Shehzad A, Wahid F, Lee YS (2010) Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 343: 489-499.
- Lopez LM (2008) Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res 52: 103-127.
- Ireson C, Orr S, Jones DJ (2001) Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res 61: 1058-1064.
- Ireson CR, Jones DJL, Orr S (2002) Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 11: 105-111.
- Rao W, Zhang W, Poventud FI (2014) Thermally responsive nanoparticle-encapsulated curcumin and its combination with mild hyperthermia for enhanced cancer cell destruction. Acta Biomater 10: 831-842.
- Sharma RA (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10: 6847-6854.
- Schneider C, Gordon ON, Edwards RL, Luis PB (2015) Degradation of curcumin: from mechanism to biological implications. J Agric Food Chem 63: 7606-7614.
- Al-Sadi RM, Ma TY (2007) IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 178: 4641-4649.
- Wang J, Ghosh SS, Ghosh S (2017) Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am J Physiol Cell Physiol 312: 438-445.
- Frisch SM, Screaton RA (2001) Anoikis mechanisms. Curr Opin Cell Biol 13: 555-562.
- Guadamillas MC, Cerezo A, Del Pozo MA (2011) Overcoming anoikis--pathways to anchorage-independent growth in cancer. J Cell Sci 124: 3189-3197.
- Sun Q, Cibas ES, Huang H, Hodgson L (2014) Induction of entosis by epithelial cadherin expression. Cell Res 24: 1288-1298.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences