ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
A Concise Review Based On Analytical Method Development and Validation Of Pregabalin
Pritam S. Jain*, Urmila R. Salunke, Santosh B. Bodkhe
Department of pharmacology, R. C. Patel Institute of Pharmaceutical Education & Research, Shirpur, india
*Corresponding author: Jain PS, Department of pharmacology, R. C. Patel Institute of Pharmaceutical Education & Research, Shirpur, india; Tel No: 919941339266; E-Mail: pritash79@yahoo.com
Received date: July 20, 2020; Accepted date: August 16, 2021; Published date: August 26, 2021
Citation:Jain PS (2020) A Concise Review Based On Analytical Method Development and Validation Of Pregabalin. Am J Pharmacol Pharmacother J Vol: 8 No: 2.
Abstract
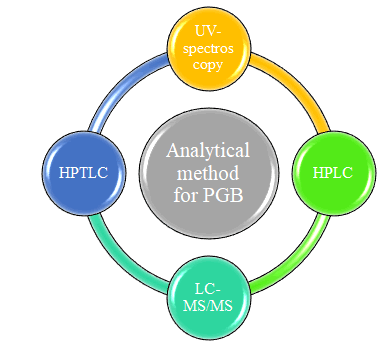
In Pregabalin is an antiepileptic drug, also called an anticonvulsant. It is the first drug which receives approved labeling from FDA for the treatment of painful diabetic neuropathy postherpetic neuralgia. It works by slowing down the impulses in the brain which causes seizures. The present critical revive assesses the completion of various article which have already been published describing analytical method and method validation for the same.The comprehensive review account, the disclosed analytical method are outlined for the establishment of pregabalin in its pharmaceutical preparations, bulk drug & biological matrices. Now days most frequently used methods such as spectrometric and liquid chromatographic method were summarized in this review. Spectrometric methods for pregabalin alone and in combination are given in Table no.1 & Table no.2.which includes parameters like λmax, solvent, matrix etc. The HPLC method for pregabalin both sole & in combination are given in Table no. 3 & 4.Includes parameters like matrix, stationary phase, mobile phase composition, detection wavelength etc. HPTLC method reported in Table no. 5 includes parameter like matrix, stationary phase, mobile phase, RF etc. The table no. 6 & 7 includes the LC-MS/MS method for pregablin for alone & in combination which involve the parameters like stationary phase, mobile phase composition, internal factor, flow rate etc.
Introduction
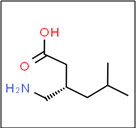
The IUPAC name of pregabalin is given as (S)-3-(aminomethyl)-5-methylhexanoic acid (Fig. 01). Pregabalin is a potent ligand for alpha- 2-delta subunit of voltage gated calcium channel in the central nervous system which shows analgesic, anticonvulsant & anxiolytic activity [1]. It is a structural analog of, but functionally dissimilar to naturally occurring transmitter GABA (Gamma aminobyuteric acid). It is generally used for the epilepsy, neuropathic pain and anxiety condition. It is soluble in aqueous solution and partially soluble in nonpolar solvent like DMSO, ethanol, DMF. It is acrystalline substance which is occur in single morphic form & it is nonhygroscopic It is thermally stable and not solvated.The molecular weight of pregabalin is 159.23g/mol and has the melting point at 186-188ºC. The compound has one stereogenic center [10].
Mechanism of action
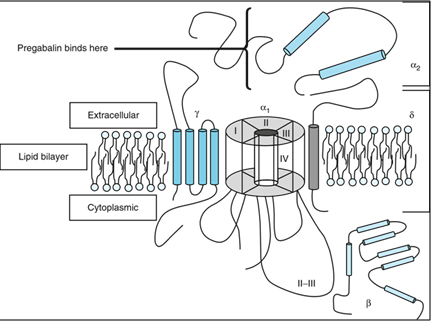
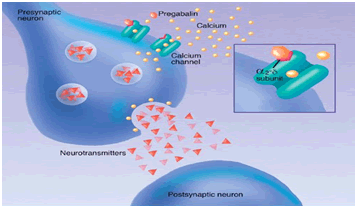
Pregabalin shows high affinity binding to the α2δ subunit of P/Q type of voltage gated channel. The voltage gated calcium channel are closed to resting membrane potential, the depolarization by action potential causes channel to open which leads the entry of Ca2+into the cell. Axonal membrane depolarizes when the action potential travels down to the neuron. When voltage gated calcium channel opens which cause intrinsic current, Neurotransmitters release from synaptic vesicle and multiplication of neurotransmission. In the presence of Ca2+exocytosis of neurotransmitter and membrane fusion occurs.`
Pregabalin target the voltage-gated calcium channel which consists of four subunit. (Fig No.2)The α1 subunit is transmembrane array forms a pores through which Ca2+ enters into cell. The α2δ subunit contains δ protein linked by a disulphide bond to α2 protein. Which have high affinity to pregabalin binding site. The β subunit is intracellular & it modifies the functioning of α2δ subunit. While the γ subunit is a glycoprotein which inline in cell membrane. [4,5]
Pragabalin pharmacokinetics
Pragabalin is quickly absorbs and shows linear pharmacokinetics after oral administration. Its oral bioavailability is ≥90% peak plasma concentration arises 1hr after oral administration& constant concentration achieve within 24-48 hrs. 20-25% peck plasma level decreases by the food intake & increase time to peak level by 3 hours. [6,8]. This studies includes the single dose and multi dose tolerance studies. [5] Pregabalin has comparatively short half-life which has volume of distribution 0.5L/kg which does not bind to plasma protein. Pharmacokinetic investigation of clinical studies shows that pharmacokinetics PGB were not significantly influence by sex or race.[6,8]
Adsorption
Pregabalin is fastly and widely absorbed after oral administration in the fasted state which shows maximum plasma concentration after 1hr in single or multiple doses and steady state being obtained within 24-48hr after repeated dosing [3]. These fast absorption properties reflect observed onset of efficacy as soon as weak one in clinical trialsperformed in patient with partial epilepsy.
Distribution, metabolism and elimination
Pregabalin is substrate of the system L carrier which is capable for the transport of large amino acid across the brain and gut. Coherent with this pregabalin can speedily cross the blood brain barrier conducted in mice during the preclinical studies which is obvious advantage for a drug that increases the CNS activity. Pregablin goes through negligible metabolism in the humans (<2%) and is excreted nearly unaffected by the kidney. Pregabalin could not bind to the plasma protein. [3]. It also not inflicts to hepatic metabolism and does not cross or restrict enzymes like cytochrome P450 system. That’s why pregabalin is improbable to cause or subject to pharmacokinetic drug-drug intraction and the anticipation that has been proved in clinical pharmacokinetic studies [9].
Analytical Accounts on Pregabalin
The widespread literature survey exposed multiple analytical techniques like UV spectrophotometry method, HPLC, HPTLC, LC-MS/MS, for the determination of pregabalin in bulk and pharmaceutical formulation. These reported method describe the evaluation of PGB in various dosage form in single constituent and in combination with gabapentin, MCA, paracetamol, methylcobalamine, mecobalamin, vigabatrin, sildenafil, amitriptyline spectracles different analytical method carry out for estimation of pregabalin.
Spectrophotometric method
Till the date numerous spectroscopic method have been accounted for the determination of pregabalin sole and in combination. This review complies the 10 papers describing spectrophotometric method for estimation of pregabalin and 2 papers for the same in combination. Table1 consist of spectrophotometric method for pregabalin and Table 2 consist of spectrophotometric method for pregabalin in combination.
Santosh G. S.et al.It can be used for the routine quality control analysis of pregabalin in bulk and pharmaceutical formulation which gives the accurate & precise method. The reportated method involves the calculation of absorbance at 210nm [11].
ARMAGAN, Onalconveyed a modest spectrophotometric scheme for estimation of pregabalin in pharmaceutical preparations. It will be determine by the three method among them first two methods were the PGB acts as n-electron donor with the DDQ and TCNQ as п acceptors which gives extremely colored compound. These compounds were determined at 494 & 841nm. The third process based on cooperation of ninhydrin with primary amine. From the three reagents TCNQ is more preferable then other two reagents based on higher molar absorptivity and lower detection limit [12].
Armagan onal, olcay sagirli The estimation of pregabalin in bulk and pharmaceutical preparation by the spectrophotometric and spectrofluorometric method pregabalin is primary amine compound which alloy which act with 7-chloro-4-nitrobenzofurazon which is extremely sensitive fluorogenic and chromogenic indicator used in many analysis. This method is relevant for routine quality control of bulk & pharmaceutical preparations without intrusion of excipients which predict to present in formulation. Spectrofluorometric method shows the higher sensitivity [13].
Rajinder Singh GujralSettled a spectrophotometric scheme for the analysis of pregablin in pure, marketed formulations and human urine sample. This process was based on reaction of drug with the blend of potassium iodate and potassium iodide. In addition this method has larger linear dynamic extent with excellent accuracy and precision. It may assist in determining influence of this drug on human being meanwhile the treatment [14]
Kaur NavneetFrom the research it is discover that the IUPAC name of pregabalin does not contain chromophoric group but it is necessary to have the chromophoric group in the structure to be UV sensitive that’s why the main objective of this research paper is to add the chromophoric group in the pregabalin structure. Which is accomplish by changing the primary amine group of pregabalin in UV sensitive product through reaction with benzyl chloride. It is concluded that throughbenzoylationmethod gives UV sensitive derivative of PGB [16]
- D. PatelOutline a spectrophotometric scheme for simultaneous estimation of multicomponent dosage form which include PGB, Methylcobalmin& alpha lipoic acid by using water as solvent system. Analysis was performed at wavelength of 436.2, 307.3, 383nm. The co-efficient co- relation found to be 99.5% for PGB, 99.56% for MCA and 99.61% ALA [20]
| Sr. No. | Compound | Matrix | Method | Detection | Solvent | Linearity | LOD & | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| (λmax) nm | LOQ | ||||||||
| 1 | PGB | Bulk & Pharmaceutical | UV spectrophotometric | 210nm | Double | 6â??14 | 2.457 mg/ml 7.448 mg/ml | 11 | |
| Dosage form | Method | distilled water | μg/ml | ||||||
| 2 | PGB | pharmaceutical preparations | UV spectrophotometric | DDQ | 494 | 2.0â??30.0 | 0.343 &1.145 | 12 | |
| Method | methanol | ||||||||
| TCNQ | 841 | Water | 1.5â??10 | 0.016 &0.055 | |||||
| Ninhydrin | 573 | DW | 40-180.0 | 1.235& | |||||
| reagent | 4.117 | ||||||||
| 3 | PGB | Bulk drug and | Spectrophotometric | 460nm | DW | 0.5â??7.0 | 0.019& | 13 | |
| Capsule | 0.0647 | ||||||||
| Spectrofluorimetric | 558nm | Chloroform | 40â??400 ngmLâ??1 | 0.049 &0.165 | |||||
| 4 | PGB | Bulk, Capsule and in Human Urine Samples | spectrophotometric method | 353nm | DW | 0.5â??3.5 | 2.46 Ã? | 14 | |
| 10â??1 | |||||||||
| 8.154 Ã? 10â??2 | |||||||||
| 5 | PGB | pure form and in capsules | spectrophotometric method | 402.6nm | Phosphate buffer pH 7.4 | 50-1000 μg mL-1 | 60& 200 μg mL-1 | 15 | |
| 6 | PGB | Pure drug & pharmaceutical formulation | UV spectrophotometric | 223nm | Methanol | 2.5-12.5 | 0.31-0.87 | 16 | |
| Method | |||||||||
| 7 | PGB | Pure form & capsule form | Spectrophotometric | 333nm | DW | 20-160 | 0.545-1.652 | 17 | |
| Spectrofluorimetric | 470nm | DW | 0.2-3 | 1.95Ã?10-3-5.9*10-3 | |||||
| 8 | PGB | Capsule dosage form | spectrophotometric method | 365nm | Water | 18-Feb | Â Â Â Â Â Â - | 18 | |
| 9 | PGB | Pure form & capsule | spectrophotometric method | 385nm | NaOH solution | 10-Feb | 0.24 & 0.74 | 19 |
Table No1: spectrophotometric method for analysis of Pregabalin
| Sr. No. | Drug | Matrix | Method | Detection | Solvent | Linearity | LOD&LOQ | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | PGB | Multicomponent dosage form | First order derivative spectroscopic method | 436.24nm | Water | 100-140 | 5.0915 & 15.4290 μg/ml | 20 |
| MCA | 307.03nm | Water | 1-1.4 | 0.01893 & | ||||
| ALA | 0.05737 | |||||||
| 383nm | Water | 130-170 | 5.4640 & 16.5576 | |||||
| 2 | pregabalin + paracetamol | Â Bulk and tablet formulation. | Â UV spectroscopic method | 210nm | Water | 14-Feb | 0.0215Â &Â 0.0651Â | 21 |
| 246nm | 0.0540 & 0.1638 |
Table No 2: Spectrophotometric for analysis of pregabalin in combination
Chromatographic overview
Apart from methods many HPLC method have been reported the determination of pregabalin in alone and in combination. In current review, a sum of 8 papers for estimation of pregabalin in sole are presented, while total sum of 7 are presented for estimation of PGB in combination are presented. The summary of reported HPLC method particularizing the mobile phase used for determination, sample matrix, λmax and linearity for PGB alone is shown in Table no. 3. While the summary of the reported HPLC method for PGB in combination is shown in table no.4.
Rajinder Singh Gujral prostrated an RP-HPLC method for the assessment of pregabalin (PGB) from bulk drug and pharmaceutical formulation. Author spent a hypersil C18 column (250mm × 4.6mm) by isocratic elusion. Mobile phase system is blend of methanol: acetonitrile: potassium hydrogen orthophosphate (3:1:16v/v/v). The main benefit of this method involve short retention time, without depletion with other reagent, stability of solvent, no requirement for the earlier separation and purification. The less chromatographic time create this method appropriate for the processing of numerous sample in definite period of time. This process can also be employ for determination of unabsorbed PGB in urine sample by very easy, cost efficient, quick and effective method. [22].
- S. Nagaraju Outlines a reverse phase HPLC scheme for evaluation of pregabalinin tablet dosage form.The mobile phase contain methanol: ammonium acetate (50:50v/v). By using phenomenex C18 column (150 × 4.6mm).The affect of acid, alkaline, photolytic stress, oxidative stress condition on PGB analyse. [23].
Reza AhmadkhanihaIn this analysis stable HPLC scheme for estimation of pregabalin in human plasma is develop. From the analysis it is concluded that the method is based on the derivatization of PGB with FDED in alkaline solution. The colored product can be found by UV detector at less concentration. From the literature review, the estimation of PGB by plasma shows that the best limit of detection was found to be 0.13μg/ml. [26].
- A. Mohansettled a RP-HPLC scheme for the concurrent estimation of pregabalin (PGB), mecobalamin, alpha lipoic acid (ALA) in capsule. Consuming an Enable make C18 column (250 × 4.6mm) as static phase and potassium dihydrogen orthophosphate buffer(balanced to pH 6 by utilizing NAOH solvent): acetonitrile: methanol (75:10:15v/v/v) as a mobile phase. The RSD value was found to be 0.84% for PGB, 1.07% for both mecobalamin and ALA.[32].
BostjanMartincsettled an analytical method for simultaneous estimation of four second generation antiepileptic drugs which include pregabalin (PGB), Gabapentin (GBP), Vigabatrin (VGB), Topiramate (TOP). Analytes were elicit from blood plasma by the help of extensive solid phase extraction derivatized with 4-chloro-7-nitrobenzofurazan and detection of HPLCwith florescence detection. The scheme is confirm acceptable for all four analytes and relevant for daily use [37].
| Sr. No. | Drug | Matrix | method | Stationary | Mobile | Detection | FR(ml/min) | RT | Rf |
|---|---|---|---|---|---|---|---|---|---|
| Phase | Phase | (nm) | |||||||
| 1 | PGB | Bulk, pharmaceutical formulation & human urine sample | RP-HPLC method | ODS hypersil column (250 mm Ã? 4.6 mm) | methanol acetonitrile - | 210nm | 1 | 5 | 22 |
| 0.02 M di - potassium hydrogen orthophosphate (K2HPO4) (pH - 7.00) (3: 1: 16, v/v/v) | |||||||||
| 2 | PGB | Pharmaceutical tablet dosage form | RP-HPLC method | Phenomenex C18 column (150 X 4.6 mm Id, ODS 2, 5μm) |  50:50 % (v/v) of Methanol & 10mM Ammonium Acetate | 210 | 0.7 | 3.39 ± 0.10 | 23 |
| 3 | PGB | In bulk/ formulation form | RP-HPLC method | Kromasil, C18, 100 x 4.6mm, 5 μm column | phosphate buffer pH 6.9 and acetonitrile (90:10) | 1 | 24 | ||
| 4 | PGB | In bulk, pharmaceutical formulation and human urine samples | RP-HPLC method | C18 5 μm ODS hypersil column (250 mm � 4.6 mm) | methanol acetonitrile - | 210 nm | 1 | 25 | |
| 0.02 M di - potassium hydrogen orthophosphate (K2HPO4) (pH - 7.00) (3: 1: 16, v/v/v) | |||||||||
| 5 | PGB | human plasma | HPLC method | TRACER EXCEL | Methanol and Na2HPO4 | 360nm | 1 | Â - | 26 |
| ODS-A stainless steel column, (5 𝜇m, 150 Ã? 4.6mm i.d., | (65:35) | ||||||||
| Teknokroma, Barcelona, Spain) | |||||||||
| 6 | PGB | in bulk drug, pharmaceutical dosage forms and human serum has | RP-HPLC | KROMASIL® 100-5 C-18 column (250�4.6 i.d. mm) | buffer pH 7 and | 210 nm | 1 | 5 | 27 |
| acetonitrile (96:4, v/v) | |||||||||
| 7 | PGB |  Bulk drugs and in capsule dosage forms. | HPLC method | Inertsil ODS -3V, C18 (250 X 4.6 mm Id, 5μm) column | 80: 10: 10 (v/v/v) of Disodium | 210nm | 1 | 5 | 28 |
| Hydrogen Phosphate Buffer: Acetonitrile: Methanol. | |||||||||
| 8 | PGB | pharmaceutical and bulk formulation | RP-HPLC Method | C18 5 μm BDS hypersil column (250 mm � 4.6 mm) | phosphate buffer | 210nm | 1 | 9 | 29 |
| solution (pH 6.9) and acetonitrile (94:6) |
Table No3: HPLC method for analysis of pregabalin
| Sr. No. | Drug | Matrix | Method | Stationary phase | Mobile pahse | Detection | FR | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | PGB + Mecobalamin + Alpha lipoic acid | In Capsule | RP-HPLC | Enable Make C18G (250 X4.6mm, 5μm) | a mixture of potassium | 210nm | 1 | 30 |
| dihydrogen orthophosphate buffermethanol & acetonitrile | ||||||||
| (75:10:15v/v) | ||||||||
| 2 | PGB+ Mecobalamin | In bulk drug & combined tab dosage form | RP-HPLC | Zodiac column 250 Χ 4.6mm | Potassium dihydrogen phosphate buffer(pH 6.5):ACN:THF(75:25:150) | 210nm | 1 | 31 |
| 3 | PGB+ methylcobalamine | In capsule | RP-HPLC | Waters allaiance 2695 | ammonium dihydrogen-o-phosphate (buffer 6.0), acetonitrile and methanol | 210 | 1 | 32 |
| seperation module,C18 column (250 Ñ? 4.6 mm,5 mcg/ml) | ( 75:15:10) | |||||||
| 4 | PGB + methylcobalamine | In capsule | RP-HPLC | Inertsil ODS 3 C-18 column | 0.01M pott. Dihydrogen & 0.01M dipotassium hydrogen phosphate: methanol(60:40) | 210nm | 1 | 33 |
| 5 | PGB+ GBP +VGB+ TOP | Human plasma | RP-HPLC | Eclipse Plus C18 column | methanol and 0.05 M phosphate buffer pH 4.9 (43:57, v/v) | 470 & 530nm | 2 | 34 |
| 6 | PGB +Methylcobalamin | In bulk &p’ceutical dosage form | RP-HPLC | C18 column | acetonitrile: methanol: ammonium acetate buffer (30:60:10) | 234nm | 1 | 35 |
| 7 | PGB +Methylcobalamin | Bulk drug and in Pharmaceutical dosage forms. | RP-HPLC | C18 column, Symmetry and Zodiac column. | Methanol:TEA Buffer: CAN | 212nm | 1 | 36 |
| 65:15:20 v/v | ||||||||
| 8 | Epalrestat and pregabalin | Tablet dosage form | RP-HPLC method | column Discovery (250 Ã? 4.6 mm) | 0.1% ortho phosphoric acid buffer and acetonitrile (45: 55 v/v) | 244nm | 1 | 37 |
| 9 | ALA, Mecobalamin and PGB | Bulk drug & combined dosage form | RP-HPLC method | Symmetry C18 (4.6 x 100mm, 5.0μm) | OPA: Acetonitrile: Methanol (60:20:20%) | 210nm | 1 | 38 |
Table No 4: HPLC method for analysis of pregabalin in combination
Hptlc Method
- B. PatilThe concurrent determination pf pregabalin and aceclofenac stability indicating method in pure and formulation. Chromatographic departure was carry out on aluminium plate smear with silica gel 60 F254 and the mobile phase was selected as toluene: methanol: formic acid (7:3:0.2v/v/v). This method all parameters were meets with the acceptable standards.[39]
Sunil More The discriminating, accurate high performance liquid chromatographic scheme for concurrent estimation of pregabalin and amitriptyline hydrochloride with densitometry in pharmaceutical preparations. The silica gel 60F254 is used as static phase and for the mobile phase toluene, methanol and formic acid (7:2.5:0.5v/v/v) is used. This scheme conclude that the establish method have many benefits like less cost consuming, relatively fast, stable, distinct, easily reproducible.[40]
| Sr. No. | Drug | Matrix | Method | Stationary phase | Mobile phase | Detection | Rf | Ref |
| 1 | Aceclofenac +Pregabaline | In bulk & in formulation | stability-indicating HPTLC | Silica Gel 60 F254 HPTLC Plate | Toluene: Methanol: Formic acid (7: 3: 0.2 v/v/v) | 210nm | 0.68 ± 0.03 (ACF)and 0.27 ± 0.03(PGB) | 39 |
| 2 | Pregabalin and Amitriptyline Hydrochloride | pharmaceutical dosage form | HPTLC | silica gel 60 F254 HPTLC method | Toluene: Methanol: Formic acid (7: 2.5: 0.5 v/v/v) | 205nm | 0.27±0.03(PGB) 0.68±0.03(AMTR) | 40 |
| Densitometry | ||||||||
| 3 | gabapentin and pregabalin | pharmaceutical dosage forms. | HPTLC method | Silica Gel G60 F254 | Ethyl Acetate: Methanol: Ammonia (6.0: 4.0: 0.1 v/v) | 210nm | 0.993(GBP) 0.992 (PBG) | 41 |
| 4 | MilnacipranHCl Duloxetine HCl and Pregabalin | Bulk drug & pharmaceutical formulation | Staility indicating HPTLC method | silica gel 60 F254 | acetonitrile-water-ammonia (6:0.6:1.6, v/v/v) | 220nm | 1 | 42 |
| dichloromethane-methanol (8:1, v/v) | 230nm | 1 | ||||||
| ethyl acetate-methanol-ammonia (6:3:0.1, v/v/v) | 210nm | 1 |
Table 5: HPTLC methods for analysis of pregabalin in combination
LC-MS/MS
LC-MS is the adaptable analytical tool which blends liquid chromatography resolving strength with mass spectrometry detection specificity. Sample compounds are isolated by liquid chromatography and then added to mass spectrometer. The mass spectrometer generate the charge ions which then tracked. The estimation of pregablin in alone and in combination is shown in table no. 6 & 7which includes different parameters like stationary phase, mobile phase, detector, internal standard etc.
- Kosticthis research paper includes determination of pregablin by novel LC-MS method in the dried matrix sport (DMS). The appealing method of sample accumulation in micro quantity was utilized in the form of dried blood sport (DBS) and dried plasma sport(DPS) followed by pre-column derivatization method. From the analysis it is concluded that the DPS is certainly can become appropriate component for all parameters using plasma matrix. Nevertheless the potential deracination of plasma by DBS depend on overcoming hematocrit issue. [43]
Pawel DzygielA simple, accurate, delicate method for simultaneous determination of pregabalin, sildenafil and active desmethyl metabolite of sildenafil. This method can be concurrently estimate by tree analyte within the in vivo concentration ranges in rat plasma. It utilizes solid-phase elicitation pursue by HPLC conjoin with mass spectrometry. It gives accuracy and precision over dyanamic ranges. [49]
| Sr.No. | Drug | Matrix | Stationary phase | Mobile phase | method | Detection\ | Discussion | IS | FR\ | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Detector | ||||||||||
| 1 | PGB | DMS | YMC-Pack Octyl column (50 x 4.0 mm, 3 μm particle size) | acetonitrile: 0.15% formic acid (85 : 15, v/v). | LCâ??MS/MS | A TSQ Quantum | Linearity: 0.200-20.0μg/mL(DBS) 0.400 â?? 40.0 μg/Ml(DPS) |  - | 550 μL/min | 43 |
| (DBS& DPS) | 104 Access MAX triple quadrupole | |||||||||
| 2 | PGB | Human plasma | Kromasil 100 C18 (3.5 μm, 3, 30 mM) column | Acetonitrile-0.5% formic acid (80:20) | LC-MS/MS | triple quadrupole mass spectrometer | 50.00 to 8003.55 ng/ml | tramadol | 1mL/min | 44 |
| 3 | PGB | Human plasma | Hypurity, 5 mm C-18 (50 ´ 4.6 mm i.d.) | buffer-methanol 20:80 (v/v) | LC-MS/MS | Biosystems MDS | 250.00 to 20000.00 ng/ml | imipramine | 0.9 ml/min | 45 |
| Sciex (API 2000) | ||||||||||
| 4 | PGB | Human plasma | Waters | formic acid and acetonitrile (30:70, v/v) | LC-MS/MS | API 4000 | 1â??10,000 ng/mL | Rosuvastatin | 1.0mL/min | 46 |
| Symmetry® C18, 100mm�4.6mm, 3.5m | triple quadrupole instrument | |||||||||
| 5 | PGB | Human plasma | Shiseido Capcell Pak MG | ammonium acetate and acetonitrile (15:85, v/v) | LC-MS/MS | API 2000 | 0.1 to 10 μg/mL | losartan | 0.2 mL/min | 47 |
| C18 column | ||||||||||
| 6 | PGB | Human plasma | ThermoHypurity C18 5 lm analytical column | acetronitrile a2 mM | LC-MS/MS | API 2000 instrument ( | 10.000â??10000.000 ng mL-1 | 1Min | 48 | |
| ammonium acetate 80:20 (v/v) |   _ |
Table 6: LC-MS/MS methods for analysis of pregabalinin combination
Conclusion
The present review elaborate various analytical approaches exercised for the appraisal of pregabalin. Numerous investigation has been performed including HPLC, HPTLC, UV-spectrometry, LC-MS/MS, GC-MS, UPLC-MS/MS etc. for the estimation of PGB in bulk drug, pharmaceutical preparations & in plasma. Further method were reported for its pharmacokinetic as well as bioequivalence studies. Few chromatography methodologies like HPLC, Stability indicating HPLC, HPTLC are also reported in literature.
References
- Ben Menachem, (2004). Pregabalin pharmacology and its relevance to clinical practice. Epilepsia, 45, pp.13-18.
- Ben-Menachem E, Kugler AR. Pregabalin and epilepsy. In: Shorvon S, Fish D, Dodson W, Perruca E, eds. Treatment of epilepsy. 2nd edition. Oxford: Blackwell Publishing Ltd, Oxford (in
- Press).
- Kugler AR, Robbins JL, Strand JC, et al. (2002) Pregabalin overview: a novel CNS-active compound with anticonvulsant activity. Poster presented at the Annual Meeting of the American Epilepsy Society, Seattle, Washington, and December.
- Yuen PW. Enantioselective synthesis of PD144723: a potent stereospecific anticonvulsant. Bioorg Med Chem Lett 1994; 4: 823-6
- Gong HC, Hang J, Kohler W, et al. (2001) Tissue-specific expression and gabapentin-binding properties of calciumchannel a2d subunit subtypes. J MembrBiol Nov;184 (1): 35-43
- Lyrica (pregabalin) package insert. New York: Pfizer; 2005 Jul.
- Perucca E, Ramsay RE, Robbins JL, Kugler AR, Anhut H, Knapp LE et al. (2002) Pregabalin demonstrates anticonvulsant activity onset by the second day. Poster presented at the Annual Meeting of the American Epilepsy Society, Seattle, and December 6â??11.
- Busch JA, Strand JC, Posvar EL et al. Pregabalin (CI-1008) single-dose pharmacokinetics and safety/tolerance in healthy subjects after oral administration of pregabalin solution or capsule dose. Epilepsia. 1998; 39:58. Abstract.
- Randinitis EJ, Posvar EL, Alvey CW, Sedman AJ, Cook JA, Bockbrader HN. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J ClinPharmacol 2003; 43:277â??83.
- US Food and Drug Administration, December 30, 2004. NDA 021446. Lyrica (pregabalin) Capsules Clinical Pharmacology Biopharmaceutics Review (s). CDER.
- Shep, S.G. and Lahoti, S.R., 2013. Development and validation of UV spectrophotometric method of pregabalin in bulk and pharmaceutical formulation. Int J Pharm Tech Res, 5, pp.1264-70.
- ARMAÄ?AN, Ã?., 2009. Development and validation of selective spectrophotometric methods for the determination of pregabalin in pharmaceutical preparation. Chinese Journal of Chemistry, 27(4), pp.781-786.
- Ã?nal, A. and Sagirli, O., 2009. Spectrophotometric and spectrofluorimetric methods for the determination of pregabalin in bulk and pharmaceutical preparation. SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy, 72(1), pp.68-71.
- Gujral, R.S., Haque, S.M. and Shanker, P, 2009. A sensitive spectrophotometric method for the determination of pregabalin in bulk, pharmaceutical formulations and in human urine samples. International journal of biomedical science: IJBS, 5(4), p.421.
- Bali, A. and Gaur, P., 2011. A novel method for spectrophotometric determination of pregabalin in pure form and in capsules. Chemistry Central Journal, 5(1), p.59.
- Navneet, K., Karan, M., Rishabh, N., Kunal, N. and Arti, T., 2010. A sensitive spectrophotometric method for the determination of pregabalin in pure drug and pharmaceutical formulations through benzoylation. Intrenational Research Journal of Pharmacy, 1(1).
- Saleh, H.M., El-Henawee, M.M., Ragab, G.H. and Mohamed, O.F., 2014. Spectrophotometric and spectrofluorimetric determination of pregabalin via condensation reactions in pure form and in capsules. International Journal of Pharmaceutical, Chemical and Biological Sciences, 4(3), pp.738-747.
- Varik, S. and Walke, T., 2013. Spectrophotometric Determination of Pregabalin from the Capsule Dosage Form Based on its Micellar Catalyzed Reaction with Sangerâ??s Reagent. International Journal of Research in Pharmaceutical and Biomedical Sciences, 4(4), pp.1051-1054.
- Rizk, M., Elshahed, M.S., Attiab, A.K. and Farag, A.S., 2015. SPECTROPHOTOMETRIC DETERMINATION OF PREGABALIN USING N-(1-NAPHTHYL) ETHYLENEDIAMINE, AS UV LABELING REAGENT. IJPBS, 5(2), pp.152-162.
- Patel, N.D., Rajyaguru, H. and Patel, P.B., 2016. DEVELOPMENT AND VALIDATION OF FIRST ORDER DERIVATIVE SPECTROPHOTOMETRIC METHOD FOR SIMULTANEOUS ESTIMATION OF PREGABALIN, METHYCOBAMIN, AND ALPHA LIPOIC ACID IN MULTICOMPONENT DOSAGE FORM. International Journal of Pharmaceutical Sciences and Research, 7(6), p.2458.
- Pawar, P.Y., Zanje, L.S., Tambe, S.S., Nandgaonkar, A.U., Funde, P.V. and Vyavhare, A.A., 2014. Simultaneous estimation of pregabalin and paracetamol by UV spectroscopic method in bulk and tablet formulation. World Journal of Pharmacy and Pharmaceutical Sciences (WJPPS), 3(4), pp.762-780.
- Gujral, R.S., Haque, S.M. and Shanker, P., 2009. A sensitive spectrophotometric method for the determination of pregabalin in bulk, pharmaceutical formulations and in human urine samples. International journal of biomedical science: IJBS, 5(4), p.421.
- Nagaraju, K.S., Rao, B.S. and Kiran, B.V., 2013. DEVELOPMENT, VALIDATION & STRESS DEGRADATION STUDIES OF PREGABALIN BY HIGH PERFORMANCE LIQUID CHROMATOGRAPHY. International Journal of Pharmaceutical Sciences and Research, 4(7), p.2782.
- Pingale, P. and Singasane, T., 2012. Development and validation of HPLC method for the determination of pregabalin in bulk and in pharmaceutical formulations. Research Journal of Pharmacy and Technology, 5(6), p.829.
- Gujral, R.S., Haque, S.M. and Kumar, S., 2009. A novel method for the determination of pregabalin in bulk pharmaceutical formulations and human urine samples. Afr J Pharm Pharmacol, 3(6), pp.327-334.
- Ahmadkhaniha, R., Mottaghi, S., Zargarpoor, M. and Souri, E., 2014. Validated HPLC method for quantification of pregabalin in human plasma using 1-Fluoro-2, 4-dinitrobenzene as derivatization agent. Chromatography Research International, 2014.
- Arayne, M.S., Shahnaz, H., Ali, A. and Sultana, N., 2014. Monitoring of pregabalin in pharmaceutical formulations and human serum using UV and RPHPLC techniques: Application to dissolution test method. Pharm Anal Acta, 5(287), p.2.
- Seema, A., Jeeja, P. and Ashish, J., 2016. Development and Validation of HPLC Method for Estimation of Pregabalin in Bulk & Capsule Dosage Form. Pharm. Anal. Acta, 7(6), pp.1-6.
- Akther, H., Morshed, M., Islam, M., Hassan, J., Barua, B.P. and Emran, T.B., 2015. Development of a Method and its Validation for Estimation of Pregabaline in Pharmaceutical and Bulk Formulation. Biomed. Sci. Today, pp.1-8.
- Mohan, A.J., Raj Kumar, B., Bhavya, T. and Ashok Kumar, A., 2014. RP-HPLC Method Development and validation for the simultaneous quantitative estimation of pregabalin, mecobalamin and alpha lipoic Acid in capsules. Int J Pharm Pharmsci, 6, p.270.
- Udayalakshmi, P., Muthukumaran, M. and Krishnamoorthy, B., SIMULTANEOUS ESTIMATION OF PREGABALIN AND METHYLCOBALAMIN BY RP-HPLC IN BULK DRUG AND COMBINED TABLET DOSAGE FORM.
- Kannapan, N., Nayak, S.P., Venkatachalam, T. and Prabhakaran, V., 2010. Analytical RP-HPLC method for development and validation of pregabalin and methylcobalamine in combined capsule formulation.
- Narmada, P., Nalini, G., Gowtham, Y., Suhasini, B. and Jogi, K.V., 2013. RP-HPLC method development and validation for the determination of methylcobalamin and pregabalin in combined capsule dosage form. International journal of research in pharmaceutical Sciences, 4(1), pp.25-29.
- Sreekanth, D., Ramya, P., Vishwanadham, Y. and Vanitha, R., 2017. Development and Method Validation of RP-HPLC For Simultaneous Determination of Pregabalin and Methylcobalamin in Pure and Pharmaceutical Dosage Form. Asian Journal of Research in Chemistry, 10(4), pp.557-565.
- Martinc, B., Roškar, R., Grabnar, I. and Vovk, T., 2014. Simultaneous determination of gabapentin, pregabalin, vigabatrin, and topiramate in plasma by HPLC with fluorescence detection. Journal of Chromatography B, 962, pp.82-88.
- Kavitha, M.P. and Rajasekhar, A., 2013. A validated HPLC method for the analysis of pregabalin and methylcobalamin in bulk and pharmaceutical formulation. PharmacieGlobale, 4(7), p.1.
- Parameswari, S.A. and Arunamma, G., 2018. STABILITY INDICATING RP-HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF EPALRESTAT AND PREGABALIN IN BULK AND TABLET DOSAGE FORM. INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH, 9(5), pp.1844-1850
- Kumar, S.A., Debnath, M., Rao, J.S. and Sankar, D.G., 2014. Stability Indicating Analytical Method Development and Validation for Simultaneous Estimation of Pregabalin, Mecobalamin and Alpha Lipoic Acid in Bulk as well as in Pharmaceutical Dosage Form by using RP-HPLC. Research Journal of Pharmacy and Technology, 7(9), p.7.
- Patil, R.B., Deshmukh, T.A. and Patil, V.R., DEVELOPMENT AND VALIDATION OF HPTLC METHOD FOR SIMULTANEOUS ESTIMATION OF ACECLOFENAC AND PREGABALIN IN BULK DOSSAGE FORM.
- More, S., Tamboli, A., Amol, V. and Patil, S., 2019. HPTLC method development for the simultaneous determination of Pregabalin and Amitryptyline hydrochloride in pharmaceutical dosage forms. Journal of Drug Delivery and Therapeutics, 9(2-s), pp.348-354.
- Prasad, M.K.C., Sagar, G.V. and Sudhakar, R.D.P., 2013. Simultaneous HPTLC Method for Estimation of Gabapentin and Pregabalin. Int. J. Pharm. Pharm. Sci, 5, pp.326-333.
- Abdel-Ghany, M.F., Abdel-Aziz, O. and WafikEskander, E., 2017. Stability-indicating HPTLC methods for determination of milnacipranHCl, duloxetine HCl, and pregabalin in bulk drug and pharmaceutical formulations. Anal ChemInd J, 17(1), p.117.
- KostiÄ?, N., Dotsikas, Y., JoviÄ?, N., StevanoviÄ?, G., MalenoviÄ?, A. and Medenica, M., 2015. Quantitation of pregabalin in dried blood spots and dried plasma spots by validated LCâ??MS/MS methods. Journal of pharmaceutical and biomedical analysis, 109, pp.79-84.
- Uma, G., Manimala, M., Vasudevan, M., Karpagam, S. and Deecarman, N., 2012. LC-MS-MS method for the determination of pregabalin in human plasma. International journal of pharmacy and pharmaceutical sciences, 4(3), pp.108-112.
- Chhabra, G.S., Bhalodiya, H.K. and Banerjee, S.K., 2012. LC-MS-MS method validation of pregabalin in human plasma. Bull. Pharm. Res, 2(2), pp.66-9.
- Nirogi, R., Kandikere, V., Mudigonda, K., Komarneni, P. and Aleti, R., 2009. Liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry method for the quantification of pregabalin in human plasma. Journal of chromatography B, 877(30), pp.3899-3906.
- Jang, K.H., Seo, J.H., Yim, S.V. and Lee, K.T., 2011. Rapid and Simple Method for the Determination of Pregabalin in Human Plasma using Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS): Application to a Bioequivalence Study of DaewoongPregabalin Capsule To Lyrica® Capsule (Pregabalin 150 mg). Journal of Pharmaceutical Investigation, 41(4), pp.255-262.
- Vaidya, V.V., Yetal, S.M., Roy, S.M., Gomes, N.A. and Joshi, S.S., 2007. LC-MSâ??MS Determination of pregabalin in human plasma. Chromatographia, 66(11-12), pp.925-928.
- Dżygiel, P. and Fraier, D., 2011. Simultaneous Determination of Pregabalin, Sildenafil and Its Active Metabolite in Rat Plasma Utilising SPE Followed by LCâ??MSâ??MS. Chromatographia, 73(11-12), pp.1177-1182.
- Merrigan, S. and Johnson-Davis, K.L., 2019. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method to Quantify Gabapentin and Pregabalin in Urine. In LC-MS in Drug Analysis (pp. 119-127). Humana Press, New York, NY.
- Validated Method for the Screening and Quantification of Baclofen, Gabapentin and Pregabalin in Human Post-Mortem Whole Blood Using Protein Precipitation and Liquid Chromatographyâ??Tandem Mass Spectrometry.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences