ISSN : ISSN: 2576-1455
Journal of Heart and Cardiovascular Research
A Case of Left Ventricular Pseudoaneurysm
Mahmoud Abdelnaby1*, Abdallah Almaghraby1, Yehia Saleh1, Basma Hammad2, Ashraf El-Amin1, Sara El-Fawal3 and Mohamed Sanhoury1
1Department of Cardiology, University of Alexandria, Egypt
2Massachusetts General Hospital, Boston, USA
3Department of Radiology, University of Alexandria, Egypt
- *Corresponding Author:
- Abdallah Almaghraby
Department of Cardiology
University of Alexandria, Egypt
Tel: +2035921675
E-mail: dr.maghraby@gmail.com
Received Date: 06 April 2017; Accepted Date: 27 April 2017; Published Date: 04 May 2017
Citation: Abdelnaby M, Almaghraby A, Saleh Y, et al. A Case of Left Ventricular Pseudoaneurysm. J Heart Cardiovasc Res 2017, 1: 1.
Copyright: © 2017 Abdelnaby M, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Pseudoaneurysm of the left ventricle is a rare devastating complication of acute myocardial infarction. It is caused by contained myocardial free wall rupture within the pericardium. We were confronted with a case of thrombus-containing ventricular pseudoaneurysm which was incidentally discovered several months after an attack of acute chest pain. The diagnosis was obtained using transthoracic echocardiography (TTE) and confirmed using cardiac magnetic resonance imaging (CMR). Unfortunately, the patient passed away before surgical intervention. This case demonstrates the importance of prompt diagnosis and management of such lethal complication. Keywords: pseudoaneurysm; pericardium; ventriculography; coronary artery
Keywords
Pseudoaneusm; Periadium; Coronary artery
Introduction
Left ventricular (LV) pseudoaneurysm is a catastrophic complication of acute myocardial infarction that is reported in less than 0.1% of patients. It is caused by myocardial rupture contained within the pericardium and is characterized by the absence of true myocardial tissue in its wall unlike, a true aneurysm which involves the full myocardial wall thickness [1]. The non-specific clinical presentation of such condition is what makes its diagnosis a clinical challenge [2]. Early diagnosis is mandatory using noninvasive modalities such as TTE and CMR or less commonly used nowadays invasive modalities such as coronary arteriography and left ventriculography [1]. Due its high liability for fatal rupture urgent surgical intervention is of paramount importance in management of this pathology [3].
Case Presentation
A 57-year-old male patient, heavy smoker, with a medical history of diabetes mellitus and hypertension and no past surgical history. He complained of exertional chest pain in the past six months associated with dyspnea grade II. In addition, he recalled an attack of acute retrosternal chest pain that lasted for several hours and was relieved spontaneously several months ago. He was not compliant on any medications and did not seek any medical advice in the past 2 years.
He presented to our facility complaining of acute chest pain. Upon examination, the patient had stable vital signs and an unremarkable clinical examination except for distant heart sounds on auscultation. Electrocardiogram showed deep Q waves in V1 to V4 with residual ST segment elevation. Routine laboratory investigations were unremarkable including serial negative troponin.
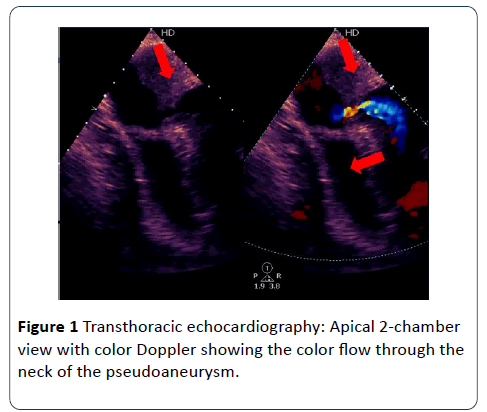
A Chest X-ray showed an enlarged cardiac shadow. TTE revealed a poor left ventricular systolic function due to an akinetic anterior wall and a dyskinetic apical cap containing a large mural thrombus. In addition to ruptured myocardium causing a large pseudoaneurysm. Also, there was a moderate circumferential pericardial effusion with no signs of cardiac tamponade.
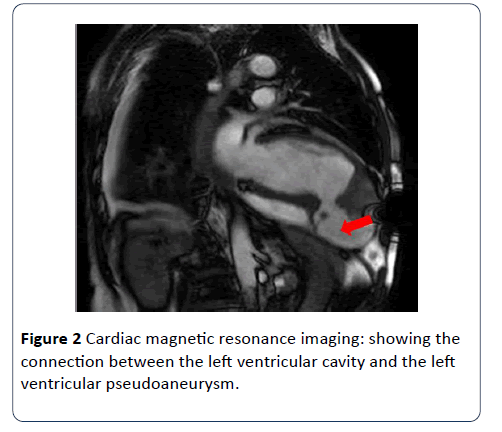
Aspirin, Clopidogrel, Enoxaparin, Atorvastatin, Furosemide and Ramipril were initiated. Urgent coronary angiography revealed a totally occluded Left anterior descending (LAD) artery and a tight lesion in the right coronary artery (RCA). Subsequently, CMR revealed a dilated left ventricle with poor systolic function, akinetic anterior wall, dyskinetic apex containing a huge thrombus measuring 34 × 33 mm, in addition to an apico-inferior outpouching consistent with a pseudoaneurysm. There was a significant amount of heterogeneous pericardial effusion with irregular fibrinous strands seen within. Late Gadolinium enhancement revealed a non-viable LAD territory.
The team decided to proceed to surgery in order to graft the RCA in addition to the pseudoaneurysm repair. Unfortunately, the patient suddenly became disoriented while having stable vital signs. A non-contrast brain computed tomography was unremarkable, after a couple of hours the patient developed asystole.
Discussion
Our case represents the dilemma of anticoagulation in patients with cardiac pseudoaneurysm, the decision should weigh the benefit of reduced thromboembolism against the risk of bleeding or pseudoaneurysm rupture. Although triple therapy was initiated, that did not prevent the patient from developing a massive stroke and hence ending his life.
Frances et al. reviewed data from 290 patients and reported that the most common etiology of LV pseudoaneurysm is myocardial infarction followed by cardiac surgery [3]. If the etiology was a myocardial infarction those patients usually present with LV pseudoaneurysm within 2 months of the event [4]. The risk factors for the LV pseudoaneurysms are old age, female sex, hypertension and inferior and lateral wall involvement in the myocardial infarction. The common location for LV pseudoaneurysm is postero-inferior followed by postero-lateral and anterior, in contrast to a true LV aneurysm which is more commonly located in the anterior and apical walls [3].
Although it is difficult to diagnose LV pseudoaneurysm due to its non-specific clinical presentation, prompt diagnosis is essential as LV pseudoaneurysm is associated with a cardiac rupture risk of 30-45%. Therefore, cardiac imaging plays a very important role in the diagnosis of LV pseudoaneurysm [2].
Due to its wide availability, TTE has increased the number of diagnosed LV pseudoaneurysm cases. Being a noninvasive technique, it is routinely used for initial assessment in suspected patients as it helps in diagnosing LV pseudoaneurysm and also in determining the extent of infarction. A ratio of <0.5 between the width of the neck and the maximal internal diameter of the aneurysmal sac or the presence of bidirectional turbulent flow through the neck by color and pulsed Doppler study are suggestive of an LV pseudoaneurysm [5,6]. TTE further assists in the improvement of the accuracy of diagnosing LV pseudoaneurysm if the TTE views are limited [6].
With the recent developments in cardiovascular imaging, CMR became one of the non-invasive modalities that enable the diagnosis of LV pseudoaneurysm, as well as its usefulness in the detection of thrombi and assessment of myocardial viability. CMR being a non-invasive modality without risk of radiation exposure, has a very important role in differentiating between LV true aneurysms and LV pseudoaneurysms, with a sensitivity of 100% and a specificity of 83% [7]. Cardiac computed tomography (CT) is another non-invasive imaging modality used for diagnosis of cardiac aneurysms but due to its limited temporal resolution, the usage of contrast and its radiation risk became less favorable [8]. Though left ventriculography is considered the gold standard modality for the diagnosis of aneurysms, it is rarely used because of the risk for thrombus dislodgement [9].
Once the diagnosis is confirmed, urgent surgical intervention is necessary in acute LV pseudoaneurysm as the risk of rupture outweighs the risk of surgery [10]. However, Small retrospective studies have shown that patients with incidental finding of chronic small LV pseudoaneurysm less than 3 cm in size and patients with increased surgical risk can be managed conservatively [10,11] (Figures 1 and 2).
Conclusion
Cardiac pseudoaneurysm is a devastating disease that rarely complicates myocardial infarctions. High clinical suspicion and the use of non-invasive imaging techniques allow early diagnosis thus reduce the risk of its fatal complications
References
- Alapati L, Chitwood WR, Cahill J, Mehra S, Movahed A (2014) Left ventricular pseudoaneurysm: A case report and review of the literatureWJCC 2:90-93.
- Contuzzi R, Gatto L, Patti G, Goffredo C, D’Ambrosio A, et al. (2009) Giant left ventricular pseudoaneurysm complicating an acute myocardial infarction in patient with previous cardiac surgery: A case report. J Cardiovasc Med (Hagerstown)10:81–84.
- Frances C, Romero A, Grady D (1998) Left ventricular pseudoaneurysm. J Am Coll Cardiol 32:557-561.
- Yeo TC, Malouf JF, Reeder GS, Oh JK (1999) Clinical characteristics and outcome in postinfarctionpseudoaneurysm. Am J Cardiol 84:592-95.
- Gatewood RP, Nanda NC (1980) Differentiation of left ventricular pseudoaneurysm from true aneurysm with two dimensional echocardiography. Am J Cardiol 46:869-878.
- Sutherland GR, Smyllie JH, Roelandt JR (1989) Advantages of colour flow imaging in the diagnosis of left ventricular pseudoaneurysm. Br Heart J 61:59-64.
- Gill S, Rakhit DJ, Ohri SK, Harden SP (2011) Left ventricular true and false aneurysms identified by cardiovascular magnetic resonance. Br J Radiol 84:35-7.
- Ghersin E, Kerner A, Gruberg L, Bar-El Y, Abadi S, et al (2007) Left ventricular pseudoaneurysm or diverticulum: differential diagnosis and dynamic evaluation by catheter left ventriculography and ECG-gated multidetector CT. Br J Radiol 80:209.
- Figueras J, Cortadellas J, Domingo E, Soler-Soler J (2001) Survival following self-limited left ventricular free wall rupture during myocardial infarction. Management differences between patients with or without pseudoaneurysm formation. Int J Cardiol 79:103-111.
- Prêtre R, Linka A, Jenni R, TurinaMI (2000) Surgical treatment of acquired left ventricular pseudoaneurysms. Ann ThoracSurg 70:553-557.
- Yeo TC, Malouf JF, Oh JK, Seward JB (1998) Clinical profile and outcome in 52 patients with cardiacpseudoaneurysm. Ann Intern Med 128:299–305.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences