ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
1’-Acetoxychavicol Acetate Accelerates Cutaneous Wound Healing by Promoting Migration of Epidermal Keratinocytes and Production of Type I Collagen Synthesis in Skin Fibroblasts
Mori A1, Sugawara K2, Oboshi M1, Matsui-Yuasa I1, Tsuruta D2 and Kojima-Yuasa A1*
1Department of Food and Human Health Sciences, Graduate School of Human Life Science, Osaka City University, Osaka, Japan
2Department of Dermatology, Osaka City University Graduate School of Medicine, Osaka, Japan
- *Corresponding Author:
- Kojima-Yuasa A
Department of Food and Human Health Sciences
Graduate School of Human Life Science
Osaka City University, Osaka, Japan
Tel: +81666052865
Fax: +81666052810
E-mail: kojima@life.osaka-cu.ac.jp
Received Date: August 31, 2018; Accepted Date: September 17, 2018; Published Date: September 20, 2018
Citation: Mori A, Sugawara K, Oboshi M, Matsui-Yuasa I, Tsuruta D, Kojima-Yuasa A et al. (2018) 1’-Acetoxychavicol Acetate Accelerates Cutaneous Wound Healing by Promoting Migration of Epidermal Keratinocytes and Production of Type I Collagen Synthesis in Skin Fibroblasts. Am J Phytomed Clin Ther Vol.6 No.3:12. DOI: 10.21767/2321-2748.100348
Abstract
Objective: 1’-Acetoxychavicol acetate (ACA), a compound contained in the rhizome and seeds of Alpinia galanga, has various physiological effects. To identify the effect of ACA on wound closure in an in vitro model of human keratinocytes and fibroblasts, and on human skin in an ex vivo wound model.
Methods: Wound closure was measured by scratch assay. The proteins were measured using by Western blot, immunofluorescence and immunohistochemical analyses. The assessment of re-epithelialization on human skin was measured using ex vivo wound model.
Results: ACA induced keratinocyte migration and wound closure. ACA also improved re-epithelialization in human skin ex vivo wounds. The expression of the cytokeratin 6 was significantly suppressed by ACA treatment. Furthermore, the present results suggest that the signaling pathway of phosphoinositide 3-kinase plays a key role in the stimulation of wound healing with ACA treatment. ACA also enhanced collagen I and elastin expressions in human skin fibroblasts and increased the secretion of bioactive transforming growth factor β which promotes cell migration in keratinocytes.
Conclusions: ACA coordinates 1) fibroblast proliferation, 2) collagen accumulation, 3) keratinocytes migration and 4) re-epithelialization, which are important in the control of wound healing. This finding suggests that ACA has clinical applications in accelerating wound healing.
Keywords
1’-Acetoxychavicol acetate; Wound healing; Keratinocyte; Skin fibroblasts; Re epithelialization
Introduction
Surgical incisions and minor lacerations, diabetic, venous, and pressure ulcers are the most common wounds. Among them, many surgical incisions and lacerations are categorized as acute wounds and almost of them heal with minimal complications. On the other hand, diabetic, venous, and pressure ulcers are chronic wounds that are difficult to heal and require expensive treatments. Recently, chronic wounds, which are commonly found in patients who are bedridden or diabetic, have become a social problem. Therefore, wound healing is important for both the prevention of infections and the improvement of the patient’s quality of life. New drugs for treating chronic wounds, including intractable skin ulcers and decubitus ulcers, have been developed by many researchers. However, unwanted side effects sometimes prevent the utilization of these new drugs. As sources for the development of new drugs, natural products are very suitable because they have no side effect. Recently, some natural products such as honey [1], aloe [2], herbs [3] and caffeine [4] have been incorporated into wound treatments. Ginger extract has also been reported to be effective in treating wounds [5]. 1’-Acetoxychavicol acetate (ACA) is a compound contained in the rhizome of Alpinia galanga and Alpinia conchigera of the ginger family, but not in other ginger. Alpinia galanga and Alpinia conchigera are distributed in Southeast Asia and are mainly used in cuisine of their countries. On the other hand, we have reported that ACA has various physiological effects, including anti-cancer [6], anti-obesity [7], antioxidant [8], detoxification of xenobiotics [9] and anti-cognitive [10]. Furthermore, other researchers have reported that ACA inhibited skin tumorigenesis in K5.Stat 3C mice, which is a model of psoriasis induced by suppressing phospho-p65 NF-β activation [11]. ACA also inhibited phorbol esters-induced oxidative stress in mouse skin and inflammatory responses [12]. However, it is not clear that whether ACA is effective on wound healing. The wound healing process depends on many types of cells. The migration, proliferation, and differentiation of keratinocytes and fibroblasts are very important for re-epithelialization and wound healing. Therefore, we examined the effect of ACA on wound closure in an in vitro human keratinocytes model, the activation of human fibroblasts, and the progression of re-epithelialization on human skin ex vivo wounds.
Materials and Methods
Culture of HaCaT cell line
HaCaT cells, the spontaneously immortalized human keratinocyte were obtained from CLS Cell Lines Service (GmbH, Eppelheim, Germany) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS), penicillin (50 units/ ml), and streptomycin (50 mg/ml).
Culture of human skin fibroblasts cell line
CCD-1059SK, human skin fibroblasts from DS Pharma Biomedical Co., LTD. (Osaka, Japan) were cultured in DMEM with 10% FBS, penicillin (50 units/ml), and streptomycin (50 mg/ml).
MTT assay
The MTT [3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] assay was carried out according to our previous report [13].
In vitro wound healing assay
In vitro wound healing assay was carried out according to Torre et al [14]. The relative migration of the cells was measured using Image J software (Wayne, Rasband, National Institutes of Health).
Transwell migration assay
Transwell migration assay was carried out according to Wolczyk et al. [15,16]. The cells were treated with 1 μM ACA and then incubated for 48 h. The cells that migrated to the lower surface of the membrane were fixed with methanol, stained with hematoxylin, and examined using an Olympus IX-70 microscope. Five randomly chosen fields were counted using ImageJ software.
Western blot analysis
Western blot analysis was carried out according to our previous report [6]. An anti-phosph-ERK1/2 antibody (Cell Signaling Technology, Inc., Danvers, MA, USA) as a primary antibody was dissolved in Can Get Signal Immunostain Immunoreaction Enhance Solution A (TOYOBO, Osaka, Japan). Biotinylated goat anti-rabbit immunoglobulin as a secondly antibody was dissolved in Can Get Signal Immunostain Immunoreaction Enhance Solution B. Then the membrane was incubated with horseradish peroxidase-coupled streptavidin. EZ West Lumi was used for color development and Densitograph Software Library CS Analyzer ver. 3.0 (ATTO Corporation, Tokyo, Japan) was used for densitometric analysis of the protein bands.
Immunofluorescence analysis
The antibody rabbit anti-phospho-AKT (Cell Signaling Technology, Inc., Danvers, MA, USA) or rabbit anti-phospho-p44/42 (Cell Signaling Technology, Inc., Danvers, MA, USA) was used as a primary antibody. The antibody goat anti-rabbit Alexa Fluor 488 (Thermo Fisher Scientific K.K., Yokohama, Japan) was used as a secondary antibody. The cells were observed under a FSX 100 Bio Imaging Navigator, which is an all-in-one fluorescence imaging system (Olympus Corporation, Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry was carried out according to our previous report [16]. Anti-collagen I polyclonal antibody (Millipore Corporation, Temecula, CA, USA) or anti-elastin polyclonal antibody (Cosmo Bio Co., Ltd, Tokyo, Japan) was used as a primary antibody. The antibody biotinylated anti-rabbit goat was used as a secondly antibody. Then the cells were incubated with horseradish peroxidase-labelled streptavidin-biotin complex. 3,3’-Diaminobenzidine tetrahydrochloride with nickel chloride was used for peroxidase reaction. The cells were observed under an Olympus IX-70 microscope.
Assay for transforming growth factor-beta (TGF β) levels in the culture medium
TGF β levels in the culture medium were analyzed using rat TGF Quantikine ELISA (enzyme-linked immunosorbent assay) Kit (R&D systems, Minneapolis, MN, USA).
Preparation of human skin for assay
Skin biopsies were obtained from three patients (23, 55, and 80 years old, all males) attending the Osaka City University Medical School Hospital. Ethical approval was done by the Institutional Research Ethics Committee of the Osaka City University Medical School. After the removal of subcutaneous fat, skin membranes were washed several times in PBS and then cultured overnight in Williams’ E medium containing penicillin, streptomycin, hydrocortisol, insulin, and L-glutamine.
Immunohistochemical staining of Ki-67 and cytokeratin 6 (CK6)
Ki-67 expression was used as an indicator of cell proliferation. Human skin specimens were used to generate acute wounds. Acute wounds were treated by a 4-mm biopsy punch. After culturing with or without 1, 5, or 10 μM ACA for 24 h in 6-well plates, skin fragments were put into OCT compound (Sakura FineTechnical Co, Ltd, Tokyo, Japan) and stored at -80˚C.The mouse anti-human Ki-67 (Dako) or mouse anti-human CK6 antibody (Abcam) was used as a primary antibody. The antibody goat anti-mouse Alexa Flour 488 was used as a secondary antibody. Then, the slides were counterstained with 4',6-diamidino-2-phenylindole (DAPI). The slides were observed under a FS X 100 Bio Imaging Navigator. The rate of Ki-67 positive cells was measured by ImageJ software.
Skin specimen preparation for the assessment of re-epithelialization
The specimens were provided by a 23-year-old man. Skin specimen preparation for the assessment of re-epithelialization was carried out according to Meier et al. [17]. The created “punch-in-punch” skin wounds were cultured in the medium with ACA (0.1 or 1 μM) for 24 h. The skin sheets were put into in OTC compound and frozen in liquid nitrogen. The antibody mouse anti-CK6 (Abcam, Cambridge, UK) was used as a primary antibody. The antibody goat anti-mouse Alexa Flour 488 was used as a secondary antibody. Then, the slides were counterstained with DAPI. The slides were observed under a FS × 100 Bio Imaging Navigator. The length of the epithelial tongue was estmated by ImageJ software.
Statistical analysis
The data are presented as the mean ± SEM. Statistical analyses were performed using Statcel3 the usefull add-in forms on excel statistical software (OMS publishing Inc., Saitama, Japan). Significant differences in assay values were evaluated using ANOVA followed by Tukey’s test. A value of p < 0.05 indicated a statistically significant difference.
Results
Effect of ACA on HaCaT keratinocyte viability
The effect of ACA on the cell number of HaCaT keratinocytes was examined using MTT assay. The number of HaCaT keratinocytes after culturing for 24 h was slightly but significantly reduced after treatment with 5 μM of ACA, but ACA had no toxicity at concentrations of up to 1 (Figure 1). From this result, effective concentration of ACA was decided to 1 μM.
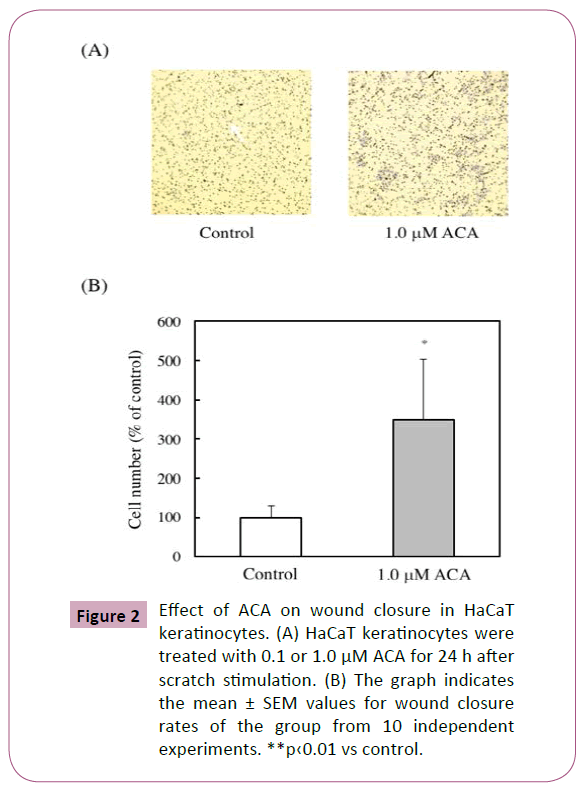
Effect of ACA on wound closure in vitro
The effect of ACA on wound closure was examined using in vitro scratch assay and evaluated the ability of migration of HaCaT cells. ACA significantly stimulated wound closure dose-dependently (Figures 2A and 2B).
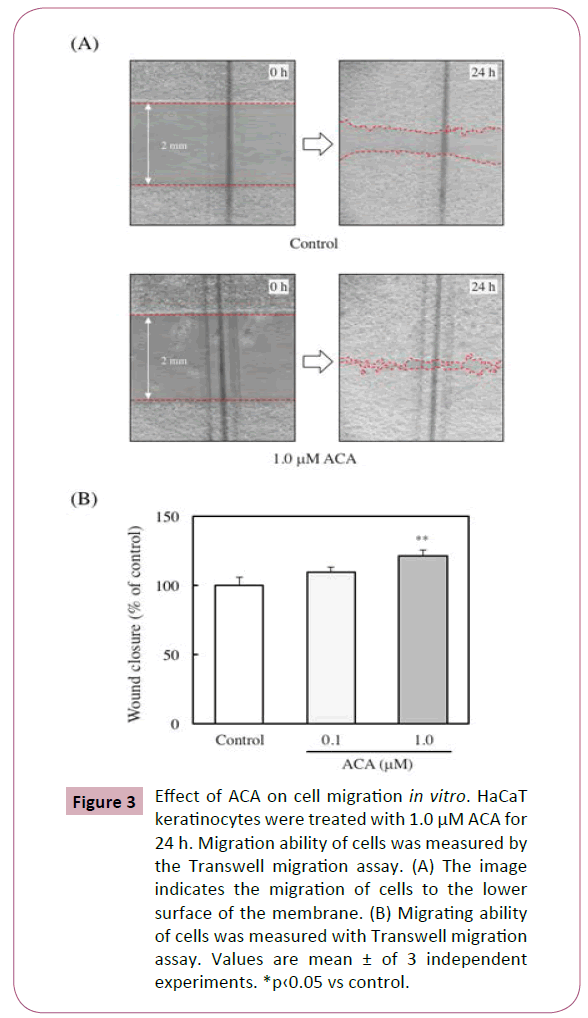
Effect of ACA on cell migration in vitro
The ability of migration of keratinocytes has been reported to be an important factor for wound healing [18]. To assess whether ACA affects the migration of HaCaT keratinocytes, the migrating ability of the cells was measured using Transwell migration assay. The migration rate of the 1 μM ACA-treated cells was about 3 folds higher than that of control cells (Figures 3A and 3B).
Figure 3: Effect of ACA on cell migration in vitro. HaCaT keratinocytes were treated with 1.0 μM ACA for 24 h. Migration ability of cells was measured by the Transwell migration assay. (A) The image indicates the migration of cells to the lower surface of the membrane. (B) Migrating ability of cells was measured with Transwell migration assay. Values are mean ± of 3 independent experiments. *p‹0.05 vs control.
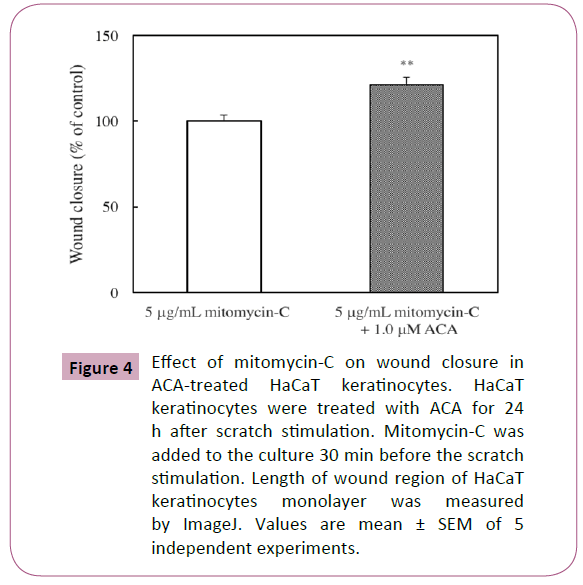
Effect of mitomycin C on wound closure in ACAtreated HaCaT keratinocytes
Gurtner et al. has suggested that the ability of keratinocyte proliferation is also an important factor for wound healing [18]. Therefore, we examined by scratch assay for the assessment of the effect of mitomycin C, an inhibitor of cell proliferation, in wound closure. However, mitomycin C did not affect the ACAinduced stimulation of wound closure (Figure 4).
Figure 4: Effect of mitomycin-C on wound closure in ACA-treated HaCaT keratinocytes. HaCaT keratinocytes were treated with ACA for 24h after scratch stimulation. Mitomycin-C was added to the culture 30 min before the scratch stimulation. Length of wound region of HaCaT keratinocytes monolayer was measured by ImageJ. Values are mean ± SEM of 5 independent experiments.
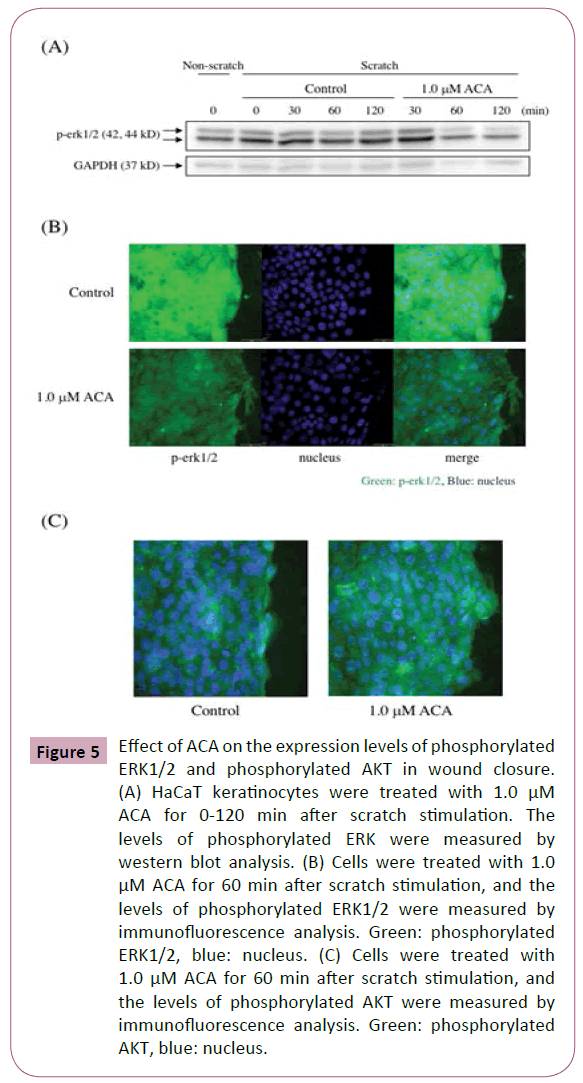
Effect of ACA on the phosphorylation of extracellular signal–regulated kinases (ERKs) and protein kinase B (AKT) in wound closure
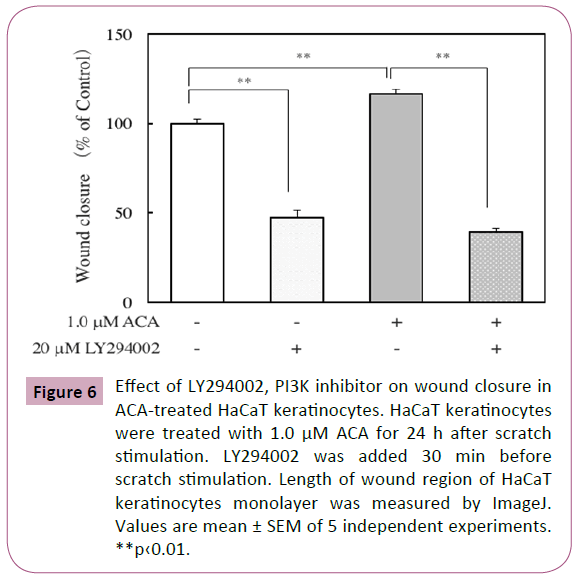
The mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) signaling pathways are involved in wound healing [19]. Here, the effect of ACA on the levels of phosphorylated ERK in scratched HaCaT keratinocytes was examined by immunofluorescence analysis and western blot analysis. After scratch stimulation, HaCaT keratinocytes were treated with or without 1.0 μM ACA. As shown in Figure 5A, immunofluorescence analysis of ACA-treated keratinocytes revealed a weaker staining for phosphorylated ERKs than that of control keratinocytes. Western blot analysis of protein lysates from scratched and ACA-treated keratinocytes also revealed reduced expression of phosphorylated ERKs (Figure 5B). Immunofluorescence analysis of ACA-treated keratinocytes revealed a stronger staining for phosphorylated AKT than that of control keratinocytes (Figure 5C). Furthermore, we examined the effect of LY294002, a PI3K inhibitor on wound closure in ACA-treated keratinocytes. The inhibitor suppressed the wound closure in both control and ACA-treated keratinocytes, but the suppression in ACA-treated cells was stronger than that in control cells (Figure 6).
Figure 5: Effect of ACA on the expression levels of phosphorylated ERK1/2 and phosphorylated AKT in wound closure. (A) HaCaT keratinocytes were treated with 1.0 μM ACA for 0-120 min after scratch stimulation. The levels of phosphorylated ERK were measured by western blot analysis. (B) Cells were treated with 1.0 μM ACA for 60 min after scratch stimulation, and the levels of phosphorylated ERK1/2 were measured by immunofluorescence analysis. Green: phosphorylated ERK1/2, blue: nucleus. (C) Cells were treated with 1.0 μM ACA for 60 min after scratch stimulation, and the levels of phosphorylated AKT were measured by immunofluorescence analysis. Green: phosphorylated AKT, blue: nucleus.
Figure 6: Effect of LY294002, PI3K inhibitor on wound closure in ACA-treated HaCaT keratinocytes. HaCaT keratinocytes were treated with 1.0 μM ACA for 24 h after scratch stimulation. LY294002 was added 30 min before scratch stimulation. Length of wound region of HaCaT keratinocytes monolayer was measured by ImageJ. Values are mean ± SEM of 5 independent experiments. **p‹0.01.
Effect of ACA on the expression levels of type I collagen and elastin in human skin fibroblasts
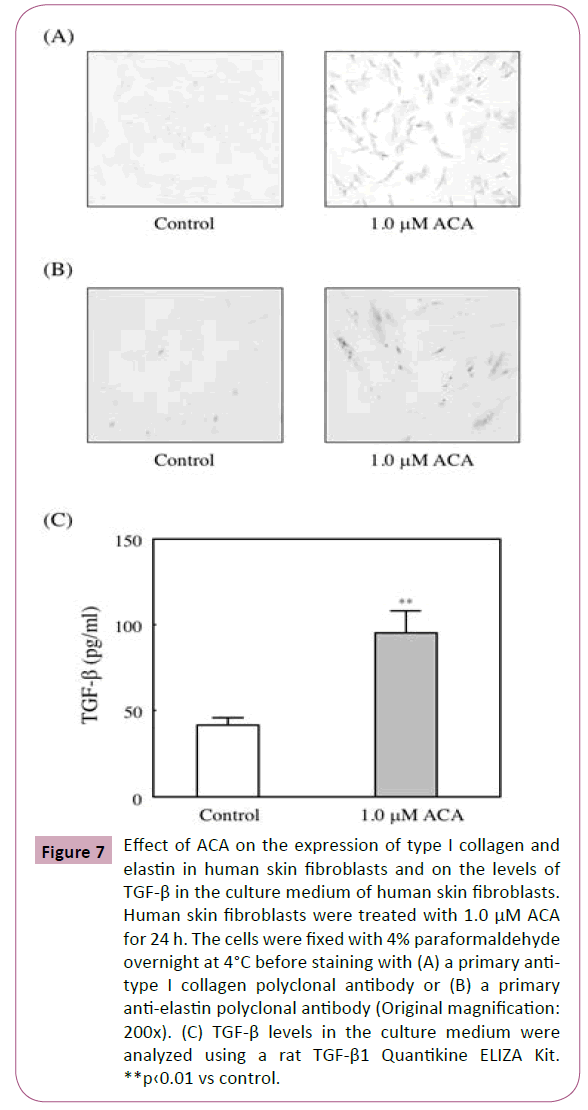
Fibroblasts play an important role in wound healing. Because they synthesize of extracellular matrix. Therefore, CCD-1059SK were incubated for 24 h with 1 M ACA. As shown in Figures 7A and 7B, treatment with ACA enhanced the collagen I and elastin expressions.
Figure 7: Effect of ACA on the expression of type I collagen and elastin in human skin fibroblasts and on the levels of TGF-β in the culture medium of human skin fibroblasts. Human skin fibroblasts were treated with 1.0 μM ACA for 24 h. The cells were fixed with 4% paraformaldehyde overnight at 4°C before staining with (A) a primary antitype I collagen polyclonal antibody or (B) a primary anti-elastin polyclonal antibody (Original magnification: 200x). (C) TGF-β levels in the culture medium were analyzed using a rat TGF-β1 Quantikine ELIZA Kit. **p‹0.01 vs control.
Effect of ACA on the secretion of bioactive TGF-β into the culture medium of human skin fibroblasts
The effects of ACA on the secretion of bioactive TGF-β was examined. And we found that treatment with 1 μM ACA significantly increased the secretion of bioactive TGF-β into the culture medium of fibroblasts (Figure 7C).
Effect of ACA on the expression of human wound skin organ culture
To further assess the effect of ACA in vitro, human skin organ was cultured with ACA for 24 h. The concentration of ACA was decided to 1, 5, or 10 μM, because it was considered that ACA might be required higher concentration in the culture medium of skin organ than that of HaCaT keratinocytes. Then, the tissues were stained immunofluorescently using Ki-67, a marker of the proliferative activity of cells. The percentage of Ki-67 positive cells in the epidermis was not affected by ACA treatment (data not shown), suggesting that ACA does not enhance cell proliferation. This result completely fits with results in in vitro HaCaT keratinocytes.
Effect of ACA on the expression of CK6 in human skin organ culture
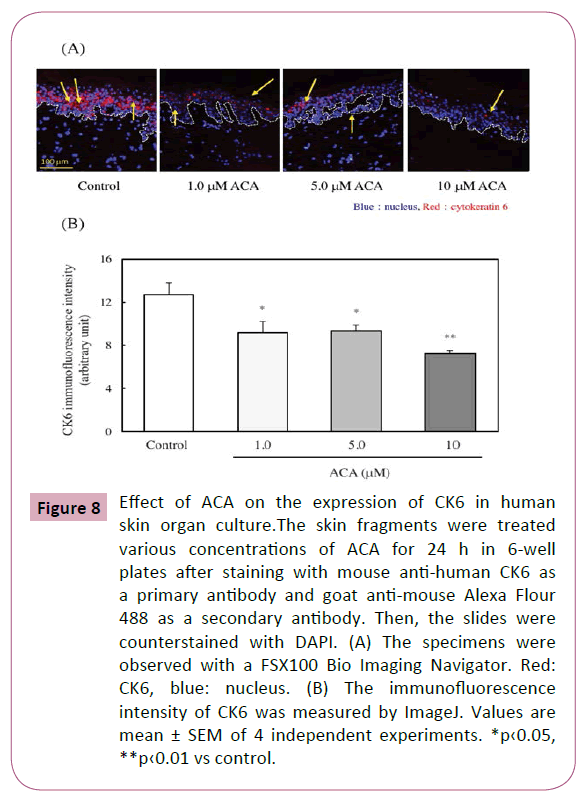
CK6 is a negative regulator for keratinocyte migration and a positive regulator for keratinocyte proliferation [20]. Here, the effect of ACA on the CK6 expression within the epidermis of organ cultured human skin was examined. As shown in Figures 8A and 8B, CK6 expression was significantly downregulated by the treatment of ACA.
Figure 8: Effect of ACA on the expression of CK6 in human skin organ culture.The skin fragments were treated various concentrations of ACA for 24 h in 6-well plates after staining with mouse anti-human CK6 as a primary antibody and goat anti-mouse Alexa Flour 488 as a secondary antibody. Then, the slides were counterstained with DAPI. (A) The specimens were observed with a FSX100 Bio Imaging Navigator. Red: CK6, blue: nucleus. (B) The immunofluorescence intensity of CK6 was measured by ImageJ. Values are mean ± SEM of 4 independent experiments. *p‹0.05, **p‹0.01 vs control.
Effect of ACA on the progression of re-epithelialization on human skin ex vivo wound model
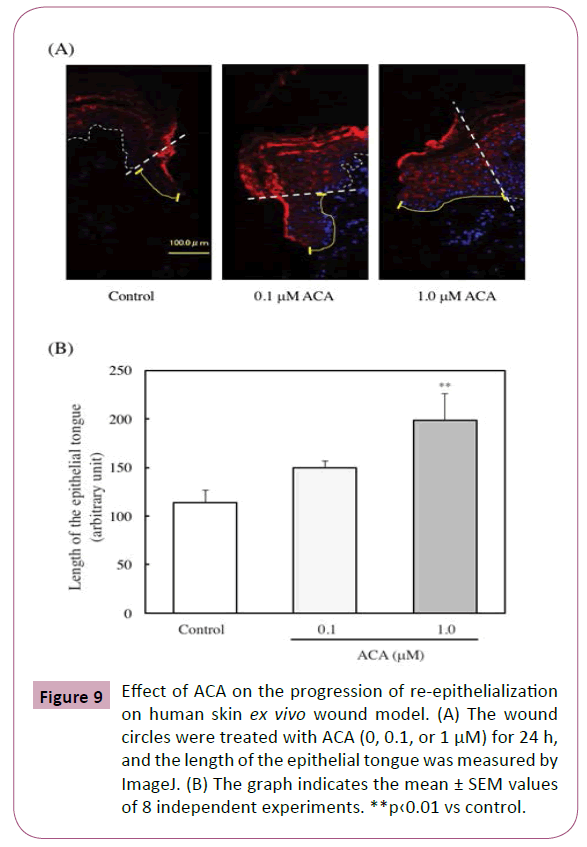
Furthermore, the effect of ACA on the progression of reepithelialization of human skin was examined. The wound circles were treated with ACA (0, 0.1, or 1 μM) for 24 h, and the length of the epithelial tongue that formed on the wound closure was then measured. As shown in Figures 9A and 9B, the epithelial tongue was significantly increased by the ACA treatment (1.0 μM).
Figure 9: Effect of ACA on the progression of re-epithelialization on human skin ex vivo wound model. (A) The wound circles were treated with ACA (0, 0.1, or 1 μM) for 24 h, and the length of the epithelial tongue was measured by ImageJ. (B) The graph indicates the mean ± SEM values of 8 independent experiments. **p‹0.01 vs control.
Discussion
The present study has shown that ACA, a compound from the rhizome and seeds of members of the ginger family, such as Alpinia galanga and Languas galanga in Southeast Asia, induces keratinocyte migration in vitro without enhancing proliferation. ACA also improved wound closure and re-epithelialization in human skin ex vivo wounds. ACA suppressed significantly the expression of CK6, an important negative regulator for keratinocyte migration. Furthermore, the present results suggest that the PI3K, but not the MAPK signaling pathway, involves in the stimulation of wound healing with ACA treatment. ACA also enhanced the expression of collagen I and elastin in human skin fibroblasts and increased the secretion of bioactive TGF-β into the culture medium of the fibroblasts.The process of wound healing depends on fibroblasts, keratinocytes and stem cells, that regulate a complex signaling network [18]. Keratinocytes epithelialize and restore the injured epidermis [21]. The migration of keratinocytes plays a key role in wound healing following injury. Sophors et al. have shown that one flavonoid, trimethoxyisoflavone, and its derivative, 2.6-dichloro-7-methoxy isoflavone, induces keratinocyte migration without affecting proliferation and then promotes wound healing [22]. Jeong et al. also reported that leucine-rich glioma inactivated 3 did not affect viability or proliferation of HaCaT keratinocyte, but stimulated the migration of the cells [23]. Recently, Yang et al. examined the involvement of proliferation or migration in wound closure using epidermal growth factor (EGF) or gallic acid (GA). EGF was suppressed the wound healing by the addition of mitomycin C, but GA was not affected by the addition of mitomycin C. These results suggested that GA promote wound healing without proliferation of in HaCaT keratinocytes [24]. Some reports have indicated that wound healing is promoted by mediating the proliferation of keratinocytes. Zhou et al. have reported that estrogentreated keratinocytes stimulates wound healing by inducing the proliferation of the cells [25]. However, the present study shows that ACA induces keratinocyte migration in wound healing and without keratinocyte proliferation. These were suggested by the lack of observed effects of mitomycin C on the ACA-induced stimulation of wound healing and ACA on DNA synthesis of keratinocytes, and the downregulation of CK6 expression by ACA treatment. Recently, Bueno et al. have shown that the crude extract of Poincianella pluviosa enhanced the migration of keratinocytes, followed by the proliferation of the cells [26]. Therefore, further studies are necessary to determine the roles of migration and proliferation in wound healing.The MAPK and PI3K/AKT signaling pathways are involved in migration in many cell types [27,28]. In the present study, we examined the effect of ACA on the levels of phosphorylated ERK and phosphorylated AKT in keratinocytes and observed the decreased phosphorylated ERK level and the increased phosphorylated AKT level in scratched and ACA-treated keratinocytes. The decreased phosphorylated ERK level was measured by immunofluorescence analysis and western blot analysis. The increased phosphorylated AKT level was also confirmed by immunofluorescence analysis and using a PI3K inhibitor, LY294002, on wound closure in keratinocytes. Huang et al. has suggested that the ERK signaling pathway, an MAPK signaling pathway, participates in cell migration [29]. Some researchers have also reported that ERK phosphorylation enhances the keratinocyte migration [30,31]. Bui et al. has shown that the increase in ERK phosphorylation was observed after treatment with 4’,6,7-trimethoxyisoflavone for 30 min, and then the ERK phosphorylation was declined. These results suggested that ERK phosphorylation is involved in switching for the other signals [32]. AKT signaling plays an important role in cell migration and proliferation [33]. However, Jeong et al. reported that leucine-rich glioma inactivated 3 stimulated the migration of human HaCaT keratinocyte through the AKT pathway [23]. Our results also indicated that ACA activated the phosphorylation of AKT and induced keratinocyte migration without enhancing proliferation. During injury, keratinocytes existing in the wound edges constitute the epithelial tongues. Then they move and reestablish coverage of the wound bed with the dermal cells and collagen-rich extracellular matrix [34]. As shown in Figures 7A and 7B, ACA treatment enhanced the expression of collagen I and elastin in fibroblasts. ACA also increased the secretion of bioactive TGF-β in the culture of fibroblasts. Armatas et al. has reported that TGF-β enhances the proliferation of adult human skin fibroblasts [35]. Rasanen and Vaheri reported that TGF-β promoted the migration of keratinocytes and inhibited the proliferation of the cells [36]. Our results indicated that the expression of CK6 was significantly downregulated by the ACA treatment in ex vivo organ cultured human skin.In conclusion, we have demonstrated that ACA coordinates 1) fibroblast proliferation, 2) collagen accumulation, 3) keratinocytes migration and 4) re-epithelialization, which are important in the control of wound healing. This finding suggests that ACA has clinical applications in stimulating wound healing.
Acknowledgments
The authors thank Dr. H. Azuma, Graduate School of Engineering, Osaka City University, who synthesized racemic ACA. This study was supported by JSPS KAKENHI Grant Numbers JP24500987, JP15K00832.
Conflict of interest
The authors declare they have no competing interests.
Source of Funding
This study was supported by JSPS KAKENHI Grant Numbers JP24500987, JP15K00832.
References
- Majtan J, Bohova J, Gracia V, Tomas FA, Masakova Z, et al. (2013) Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: a new action of honey in wound healing. Arch Dermatol Res 305: 619-627.
- Choi SW, Son BW, Son YS, YI, Lee SK, et al. (2001) The wound healing effect of a glycoprotein fraction isolated from aloe vera. Br J Dermatol 145: 535-545.
- Liu CL, Tam JC, Sanders AJ, Ko CH, Fung KP, et al. (2013) Molecular angiogenic events of a two-herb wound healing formula involving MAPK and Akt signaling pathways in human vascular endothelial cells. Wound Repair Regen 21: 579-587.
- Ojeh N, Stojadinovic O, Pastar I, Sawaya A, Yin N, et al. (2014) The effects of caffeine on wound healing. Int Wound J 13: 605-613.
- Chen CY, Chiu CC, Wu CP, Chou YT (2013) Enhancements of skin cell proliferations and migrations via 6-dehydrogingerdione. J Agric Food Chem 61: 1349-1356.
- Kato R, Matsui-Yuasa I, Azuma H, Kojima- Yuasa A(2014) The synergistic effect of 1'-acetoxychavicol acetate and sodium butyrate on the death of human hepatocellular carcinoma cells. Chem Biol Interact 212: 1-10.
- Ohnishi R, Matsui-Yuasa I, Deguchi Y, Yaku K, Tabuchi M, et al.(2012) 1'-Acetoxychavicol acetate inhibits adipogenesis in 3T3-L1 adipocytes and in high fat-fed rats. Am J Chii Med 40: 1189-1204.
- Higashida M, Xu S, Kojima-Yuasa A, Kennedy DO, Murakami A, et al.(2009) 1'-Acetoxychavicol acetate-induced cytotoxicity is accompanied by a rapid and drastic modulation of glutathione metabolism. Amino Acids 36: 107-113.
- Yaku K, Matsui-Yuasa I, Konishi Y, Kojima-Yuasa A (2012) AMPK synergizes with the combined treatment of 1'-acetoxychavicol acetate and sodium butyrate to upregulate phase II detoxifying enzyme activities. Mol Nutr Food 57: 1198-1208.
- Kojima-Yuasa A, Yamamoto T, Yaku K, Hirota S, Takenaka S, et al. (2016) 1’-Acetoxychavicol acetate ameliorates age-related spatial memory deterioration by increasing serum ketone body production as a complementary energy source for neuronal cells. Chem Biol Interact 257: 101-109.
- Battra V, Syed Z, Gill JN, Coburn MA, Adegboyega P, et al. (2012) Effects of the tropical ginger compound, 1’-acetoxychavicol acetate, against tumor promotion in K5 Stat3C transgenic mice. J Exp Clin Cancer Res 31: 1-14.
- Nakamura Y, Murakami A, Ohto Y, Torikai K, Tanaka T, et al. (1998) Suppression of tumor promoter-induced oxidative stress and inflammatory responses in mouse skin by a superoxide generation inhibitor 1'-acetoxychavicol acetate. Cancer Res 58: 4832-4839.
- Yokogawa K, Matsui-Yuasa I, Tamura A, Terada M, Kojima-Yuasa A, et al. (2011) Inhibitory effects of Ecklonia cava extract on high glucose-induced hepatic stellate cell activation. Mar Drugs 9: 2793-2808.
- Torre CL, Cinque B, Lombardi F, Miconi G, Palumbo P, et al. (2016) Nitric oxide chemical donor affects the early phases of in vitro wound healing process. J Cell Physiol 231: 2185–2195.
- Wolczyk D, Zaremba CM, Hryniewicz JA, Tabola R, Grabowski K, et al. (2016) TNF-α promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts. Cell Oncol 39: 353–363.
- Kojima-Yuasa A, Umeda K, Ohkita T, Kennedy DO, Nishiguchi S, et al. (2005) Role of reactive oxygen species in zinc deficiency-induced hepatic stellate cell activation. Free Radic Biol Med 39: 631-640.
- Meier NT, Haslam IS, Pattwell DM, Zhang GY, Emelianov V, et al. (2013) Thyrotropin-Releasing Hormone (TRH) Promotes Wound Re-Epithelialisation in Frog and Human Skin. Plos ONE 8: 1-16.
- Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453: 314-321.
- Fitsialos G, Chassot AA, Turchi L, Dayem MA, LeBrigand K, et al. (2007) Translational signature of epidermal keratinocytes subjected to in vitro scratch wound reveals selective roles for ERK1/2, p38, and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 282: 15090-15102.
- McGowan K, Coulombe PA (1998) The wound repair-associated keratins 6, 16, 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell Biochem 31: 173-204.
- Pastar L, Stjadinovic O, Yin NC, Ramirez H, Nusbaum AG, et al. (2014) Epithelialization in wound healing a comprehensive review. Adv Wound Care 3: 445-464.
- Sophors P, Kim YM, Seo GY, Huh JS, Lim Y, et al. (2016) A synthetic isoflavone, DCMF, promotes human keratinocyte migration by activating Src/FAK signaling pathway. Biochem Biophys Res Commun 472: 332-338.
- Jeong YM, Park WJ, Kim MK, Baek KJ, Kwon NS, et al. (2013) Leucine-rich glioma inactivated 3 promotes HaCat keratinocytes migration. Wound Repair Regeneration 21: 634-640.
- Yang DJ, Moh SH, Son DH, You S, Kinyua AW, et al. (2016) Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules 21: 899.
- Zhou T, Yang Z, Chen Y, Chen Y, Huang Z, et al. (2016) Estrogen accelerates cutaneous wound healing by promoting proliferation of epidermal keratinocytes via Erk/Akt signaling pathway. Cell Physiol Biochem 38: 959-968.
- Bueno FG, Moreira EA, De Morais GR, Pacheco IA, Baesso MI, et al. (2016) Enhanced cutaneous wound healing in vivo by standardized crude extract of Poincianella pluviosa. Plos ONE 11: 1-13.
- Segarra J, Balenci L, Drenth T, Maina F, Lamballe F (2006) Combined signaling through ERK, PI3K/AKT, and RAC1/p38 is required for Met-triggered cortical neuron migration. J Biol Chem 281: 4771-4778.
- Du J, Sun C, Hu Z, Yang Y, Zhu Y, et al. (2010) Lysophosphatidic acid induced MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. Plos ONE 5: 1-10.
- Huang C, Jacobson K, Schaller MD (2004) MAP kinases and cell migration. J Cell Sci 117: 4619-4628.
- Shi I, Zeng X, Zhou Z, Chen Q (2009) Activation of ERK-FAK signaling pathway and enhancement of cell migration involved in the early interaction between oral keratinocytes and Candida albicans. Mycopathologia 167: 1-7.
- Wahedi HM, Park YU, Moon EY, Kim SY (2016) Juglone ameliorates skin wound healing by promoting skin cell migration throgh Rac/Cdc42/PAK pathway. Wound Repair Regeneratin 24: 786-794.
- Bui NT, Ho MH, Kim YM, Lim Y, Cho M (2014) Flavonoids promoting HaCat migration: II. Molecular mechanism of 4’,6,7-trimethoxyisoflavone via NOX2 activation. Phytomedicine 21: 570-577.
- Kandel ES, Skeen I, Majewski N, Di Cristofano A, Pandolfi PP, et al. (2002) Activation of Akt/protein kinase B overcomes G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol 22: 7831-7841.
- Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49: 35-43.
- Armates AA, Paratsinis H, Mavrogonatou E, Angelopoulou MT, Kouroumalis A, et al. (2014) The differential proliferative response of fetal and adult human skin fibloblasts to TGF-beta is retained when cultured in the presence of fiblonectin or collagen. Biochim Biophys Acta 1840: 2635-2642.
- Rasanen K, Vaheri A (2010) TGF-beta1 causes epithelialmesenchymal transition in HaCaT derivatives, but induces expression of COX-2 and migration only in benign, not in malignant keratinocytes. J Dermatol Sci 58: 97-104.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences