ISSN : 0976-8505
Der Chemica Sinica
Abstract
Metal Complexes of Nicotine: A Group of Negligible Compounds
Nicotine (NIC) is an oxygenic alkaloid compound exists in two forms R and S, and consists of a pyridine ring, substituted at the 3-position with an N-methyl-pyrrolidine ring. Nicotine is extracted mostly from the Nicotiana tabacum plant, and although it was made in the roots and accumulates in smaller amounts in leaves of edible plants of the nightshade family called “Solanaceae’’ such as tomatoes, potatoes, green peppers, and coca plants. Biochemically, it’s parasympathomimetic, stimulant drug, and is a nicotinic acetylcholine receptor agonist. It functions as an antiherbivore, insecticide, imidacloprid, and recently chromatin-modifying enzymes inhibitor. Nicotine was considered to be one of the most biologically important compounds, for which information about their metal ions complexation properties are very rare in the literature. However, recently it gains much attention from many bioinorganic chemists over the world. In the present mini review article, I described the research done during the last few decades about the solid chemistry of nicotine and studies concerning a number of crystal structures of nicotine complexes. Also, I reported about the rare research work done previously about the protonation and complexation equilibria studies of nicotine in which the data of the different equilibrium constants and stability constants of nicotine would be valued in metal based drug research.
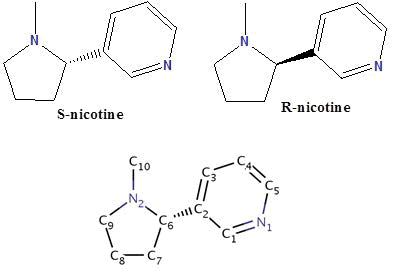
Graphical Abstract: Molecular Structure of Nicotine ((S)/(R)-3-(1-Methylpyrrolidine-2-yl) pyridine, NIC).
Author(s): Ahmed E Fazary
Abstract | Full-Text | PDF
Share This Article
Google Scholar citation report
Citations : 6019
Der Chemica Sinica received 6019 citations as per Google Scholar report
Der Chemica Sinica peer review process verified at publons
Abstracted/Indexed in
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Directory of Research Journal Indexing (DRJI)
- Publons
- MIAR
- International Committee of Medical Journal Editors (ICMJE)
- Serials Union Catalogue (SUNCAT)
- Geneva Foundation for Medical Education and Research
- Secret Search Engine Labs
- Euro Pub
- CAS (Chemical Abstracting Services)
- University of Barcelona
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
