ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Continuous-Flow Hydrogenation of D-Xylose with Bimetallic Ruthenium Catalysts on Micrometric Alumina
1Department of Chemistry, Uppsala University, Uppsala 75120, Sweden
2Institute of Physical Chemistry, Polish Academy of Sciences, Warsaw 01-224, Poland
- *Corresponding Author:
- Jacinto Sá
Nanoleaves and Heterogeneous Catalysis group
Department of Chemistry
Ångström Laboratory
Uppsala University
Uppsala 75120, Sweden.
Tel: +46 18 471 68 06
E-mail: jacinto.sa@kemi.uu.se
Received Date: December 16, 2016; Accepted Date: January 06, 2017; Published Date: January 12, 2017
Citation: Paun C, Lewin E, Jacinto Sá. Continuous-Flow Hydrogenation of D-Xylose with Bimetallic Ruthenium Catalysts on Micrometric Alumina. Synth Catal. 2017, 2:1.
Abstract
Continuous-flow hydrogenation of D-xylose to xylitol was evaluated with pristine and silver modified ruthenium supported on micrometric alumina. The original size of alumina support is required for direct use in flow hydrogenation since it prevents setup clogging. The parent ruthenium catalyst shows high activity and selectivity to the desired product. However, there was an evident competition between adsorption of substrate and product desorption, and ruthenium nanoparticles aggregation. In situ modification of the parent catalyst with silver improved catalysts stability and minimize the competitive adsorption behavior between reactant and product.
Keywords
Hydrogenation; D-xylose; Xylitol; Ruthenium catalysts; In situ; Silver
Introduction
Biomass upgrading conveys the production of fuel and/or chemicals from bioresources. To improve the economics of any biomass conversion process, it is crucial to convert all the three fractions, i.e., convert the cellulose, hemicellulose and lignin fractions. Cellulose is by far the most used biomass fraction either as it is by the pulp/paper industry or in the chemicals and biofuel synthesis. The hemicellulose sugar content is often fermented to produce bioethanol. However, the fermentation's economic viability requires conversion of both pentoses (the most abundant units) and hexoses units. Fermentation microorganisms can convert easily hexose units, such as glucose, but the bioconversion of pentoses remains relatively inefficient. Additionally, while sugar fermentation to bioethanol can be considered a carbon neutral process since the plants reuse the CO2 produced, it is not atom efficient because a molecule of CO2 is generated per molecule of ethanol produced.
Catalytic hydrogenation of sugar units with molecular hydrogen is an atom efficient transformation, with potential to generate high value sugar alcohols, such as xylitol from xylose pentose sugar unit. Xylose is safe for use in foods, with known antibacterial, antifungal and healing properties [1]. Xylitol and arabitol are considered safe sweeteners [1] and with reported benefits to dental health [2]. Consumers increasing consciousness to health and weight, advocates for an increase in the demand for xylitol and arabitol in the upcoming decades, following the trend of all sugar-free and low-calorie food products. The global market for xylitol is expected to reach 242 thousand metric tons valued at just above US$1 billion by 2020 [3,4]. Additionally, xylitol has positive affects in the digestive process, with some studies showing that it can prevent cancer of the digestive tract [1]. Xylitol is also a big component in fighting middle ear infections in children and increase of bone density in elderly people [1].
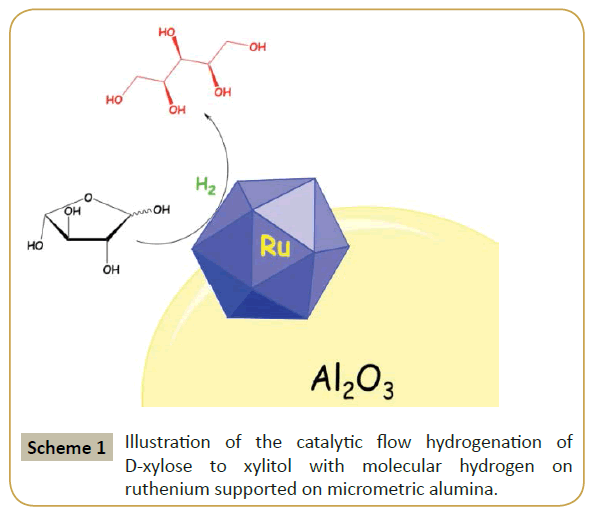
Currently, xylitol is manufactured via chemical hydrogenation of xylose in a three-phase slurry batch reactor with Raney nickel catalyst [5,6]. The use of nickel as catalyst has some major disadvantages, such as relatively fast deactivation due to organic impurities and metal leaching. The latter is a major concern due to nickel's toxicity, which precludes large-scale application by the food or pharmaceutical industries. Research in alternatives based on noble metal catalysts (Pd, Pt, Ru) has increased in the past years [7]. Special attention has been given to Ru based catalysts, tested primarily in a slurry batch reactor [8-10]. From green, economical and industrial perspectives flow processes are desirable [1], which requires development of novel and ready-to-use catalysts with suitable nominal sizes for flow applications, i.e., to prevent reactor clogging. Herein, it is reported the catalytic continuous-flow hydrogenation of D-xylose to xylitol with a ruthenium catalyst on micrometric alumina (Scheme 1). The catalyst has been prepared for its direct use in flow hydrogenation reactions. Activity, selectivity and stability were modified by in situ post-synthetic modification of the parent catalyst with silver. This preparation can be effortlessly adapted and applied for the deposition of other metals/metal-complexes bringing benefits for those who are looking into changing the steric and electronic properties of parent catalysts.
Experimental Procedures
Catalyst synthesis of ruthenium on micrometric alumina
Ruthenium on micrometric alumina (Ru/Al2O3) was synthesized by an impregnation-reduction method similar with the one reported by Mishra et al. [11]. In order to deposit Ru (3 wt%) on Al2O3 (granule size>250 μm), RuCl3·xH2O (0.02 M) was dissolved in 40 ml dry ethanol in a two neck round bottle flask with a mechanical stirrer and a nitrogen inlet. The mixture was stirred at room temperature under N2 for 24 h. Then, 0.2 M solution of NaBH4 in ethanol was added drop wise, while the solution was continuously stirred under nitrogen. The solution turned dark grey within minutes and was left to stir for additionally 24 h at room temperature. Finally, the solvent was removed with a rotary evaporator and the catalyst was washed with fresh ethanol several times. Upon drying at room temperature a grey catalyst was obtained.
Modification of the parent catalyst with silver
Post-synthetic modification of the ruthenium catalyst with silver (Ag@Ru/Al2O3) was performed by a new and easy method, in a continuous-flow in the H-Cube apparatus. Basically, the ruthenium parent catalyst was loaded into the cartridge-reactor, and a AgNO3 water solution (1.5 mM; calculated for the ratio Ag:Ru=0.6) was flown (0.3 ml/min) through the catalyst under H2 pressure (10 bar), at 80°C for 100 min. By changing the synthesis procedure from batch to continuous flow, sample homogeneity was dramatically improved. Deposition was monitored by UVvis absorption spectra of the solutions at the exit of the reactor (Supplementary Information (SI); Figures S1 and S2). The absorption spectrum of the silver nitrate precursor had two peaks (223 nm and 296 nm) in accordance with the literature [12]. However, upon passing through the catalyst, in the reaction conditions, the peak at 296 nm disappeared and the peak at 223 nm decreased in intensity. No other peaks were formed, which supports our presumption that silver is deposited in situ, further confirmed by XPS analysis.
X-ray photoelectron spectroscopy
X-ray Photoelectron Spectroscopy (XPS) was performed on a Physical electronics Quantum 2000 spectrometer, using monochromatic Al Kα radiation (hν=1486.7 eV). Both survey and higher resolution spectra of selected spectral regions were attained using an analysis spot with a diameter of 200 μm. All measurements were performed under constant charge neutralisation from very low energy electrons and Ar+ ions, ensuring stable measurement conditions [13].
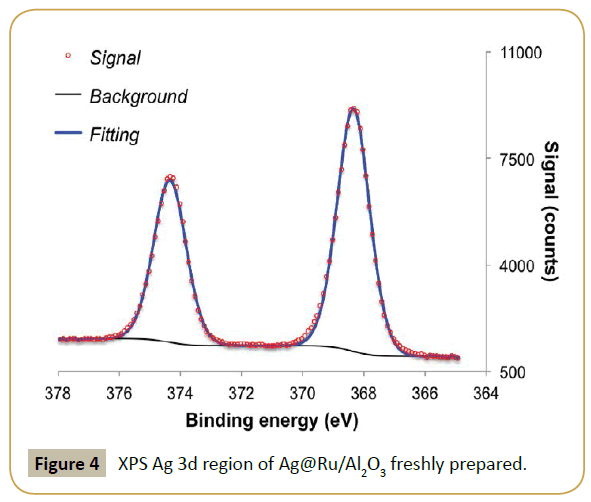
XPS data was fitted using CasaXPS package. Charge referencing was performed setting the binding energy Al 2p3/2 to 74.2 eV [14] using a double separation (DS) of 0.41 eV [15], and constrains for area and full width half maximum (fwhm). It was opted to use the Al 2p3/2 because the advantageous carbon is overlapped with Ru 3d signals. Ru 3p signals were fitted constraining the doublet area intensity ratio (A3p1/2/A3p3/2=0.5), DS=22.1 eV [16,17] and same fwhm. Ag 3d signals were fitted constraining the doublet area intensity ratio (A3d3/2/A3d5/2=0.67), DS=6.0 eV [18] and same fwhm.
Catalytic flow-hydrogenation
The reactions were performed in a continuous-flow flooded-bed micro-reactor (ThalesNano H-Cube Mini™). Typically, the so-called CatCart® (30 mm length) was filled with 250 mg of catalyst. At room temperature and ambient pressure a solution of xylose (0.1 M) was passed through the catalyst with a 2 ml/min flow, until the concentration at exit of the reactor matched the initial xylose concentration (according to Raman spectroscopy signal). Typical reaction conditions were: flow of 0.2 ml/min solution of xylose (0.1 M), 100°C and 80 bar H2. After the system reached stability with the reaction condition (within 10 min), fractions were collected at regular time intervals and analyzed by Raman spectroscopy. Blank reactions entail reactions with empty CatCart® or loaded with Al2O3 and identical reaction conditions.
Substrate and product concentration were analyzed with fiber optic Raman spectroscopy setup equipped with a 785 nm laser, a stainless steel RPS probe (probe length 76 mm, probe diameter 12.7 mm) and a QE-Pro detector, from Ocean Optics. Spectra were recorded at room temperature in a standard quartz cuvette placed in a homemade cardboard box, recorded between 250– 2000 cm-1 Raman shift using 5 s acquisitions with 10 accumulations for all measurements. The laser power for all measurements was approximately 650 mW. Calibration and Raman signal dependence in respect to concentration are presented in SI Figures S3-S6.
Results and Discussion
XPS survey scans were used to estimate the metal ratios and the changes induced by 300 min of reaction. The results are summarized in Table 1. Table 2 shows the corrected binding energies for the metals of interest before and after reaction.
| Sample | A(Ru3p3/2)/A(Al2p) | A(Ag3d5/2)/A(Al2p) |
|---|---|---|
| Ru/Al2O3 fresh | 1.7 | - |
| Ru/Al2O3 used | 0.4 | - |
| Ag@Ru/Al2O3 fresh | 0.3 | 3.5 |
| Ag@Ru/Al2O3 used | 1.2 | 3.7 |
Table 1 Area ratio estimated from XPS survey scan.
| Sample | Binding energy Ru3p3/2 (eV) | Binding energy Ag3d5/2 (eV) |
|---|---|---|
| Ru/Al2O3 fresh | 462.2 | - |
| Ru/Al2O3 used | 462.0 | - |
| Ag@Ru/Al2O3 fresh | 461.7 | 368.3 |
| Ag@Ru/Al2O3 used | 461.8 | 368.1 |
Table 2 XPS corrected binding energy for the species of interest.
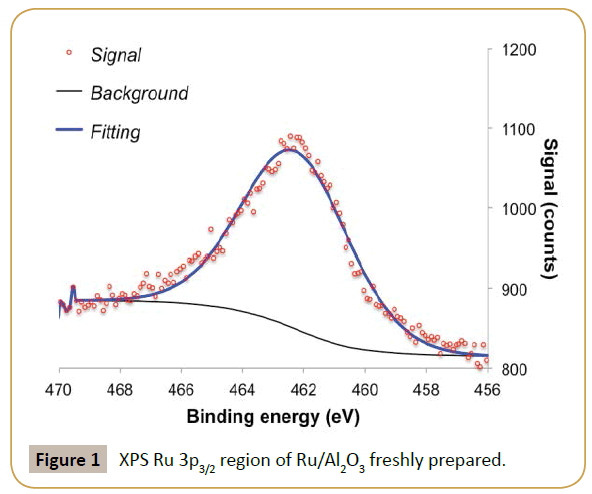
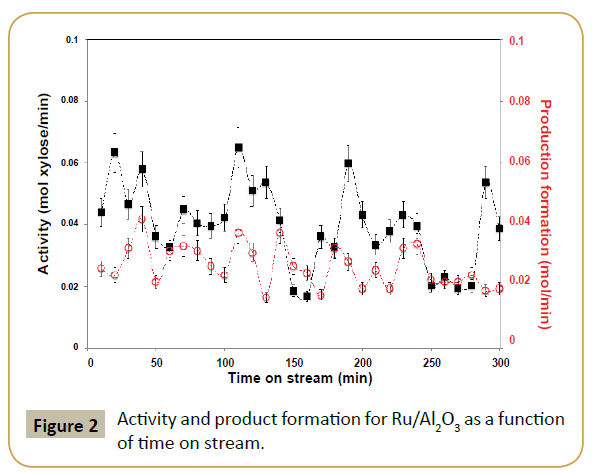
The fresh ruthenium parent catalyst had a high Ru/Al ratio (ca. 1.7), suggesting high metal dispersion. XPS analysis of Ru 3p3/2 region shows a single feature with binding energy around 462.2 eV, which was assigned to metallic ruthenium (Figure 1) [19]. The value is a bit higher in energy than most commonly reported values (Ru0 between 461.1-461.6 eV [20]) but below the values expected for RuO2 (462.4-462.5eV [21]). The observed binding energy allied to the small peak asymmetry observed at high binding energy and Ru/Al ratio suggests the presence of ruthenium as nanoparticles [22-25]. This is further corroborated by the absence of diffraction peaks associated to ruthenium in X-ray diffraction, suggesting crystallite sizes below 4 nm [24-25]. The Ru/Al2O3 catalyst was used directly in the flow hydrogenation of D-xylose to xylitol. The size of the alumina particles (>250 μm) ensured that there were no problems associated with clogging or liquid flowing through the catalyst bed. As it can be seen in Figure 2, despite the low residence times in the reactor (Approx. 70 s) good steady state conversions of xylose were recorded (Approx. 40%), equating to an average activity of about 0.04 mol/ min. Xylitol formationrate was found to be on average 0.03 mol/ min, equating to an average selectivity about 70%. It should be mentioned that the alumina support was completely inactive in this hydrogenation.
Reaction catalytic profile has an oscillatory behavior for both xylose conversion and xylitol selectivity as reaction progresses in time. XPS analysis of the catalyst post-reaction (Tables 1 and 2) suggests ruthenium particles aggregation and possibly some metal leaching since the XPS shows a decrease in Ru/Al ratio and a shift to lower binding energy of the Ru 3p3/2 peak. The shift in binding energy suggests less contact between ruthenium and alumina support consistent with formation of larger particles. Additionally, the catalytic results suggest that there is a competition between substrate adsorption and product desorption, resulting in a characteristic oscillatory behavior of the reaction profiles as observed. This also justifies why in some cases the amount of xylitol produced is larger than xylose consumed. It is known that during xylose hydrogenation small amounts of by-products can also be formed. However, the reaction conditions in which we are operating the reactor (low temperature and high hydrogen pressure) would prevent the formation of such by-products on a ruthenium catalyst according to literature [11]. Thus, by-product formation is not the reason for the oscillatory behavior.
In order to diminish the competitive effect between xylose adsorption and xylitol desorption, a second metal, in this case silver, was added to the catalyst. The hope was that the added second metal could alter the electronic and/or geometric structure of the parent catalyst. Metallic silver has a 4d10 electronic structure, which should donate electron density to the metallic ruthenium (4d7). According to Nørskov’s model, catalysis is fundamentally controlled by the catalyst electron occupancy and energy of valence shell orbitals [26-30], i.e., filling of the d-band.
Cheng et al. [31] doped a ruthenium catalyst with SnOx and reported an electronic interaction between Ru-SnOx due to an electron transfer from the Ru nanoparticles to SnOx, inducing an electron deficit state of ruthenium nanoparticles, which was favorable for the adsorption of the electropositive N-atom belonging to the reactant nitro group. The electron deficient state of ruthenium can also be favorable for the hydrogen adsorption, resulting in the production of more active hydrogen species.
Silver was deposited directly on the catalyst placed in the CatCart holder, which would be subsequently used in the hydrogenation reaction. The concentration of the silver could be easily varied by the initial AgNO3 precursor concentration, and the conditions used for deposition were mild, so that no other changes could be introduced in the parent catalyst. It should be noted that this procedure could be adapted for the deposition of any other element by simply choosing a reducible salt or complex of the element of interest [30].
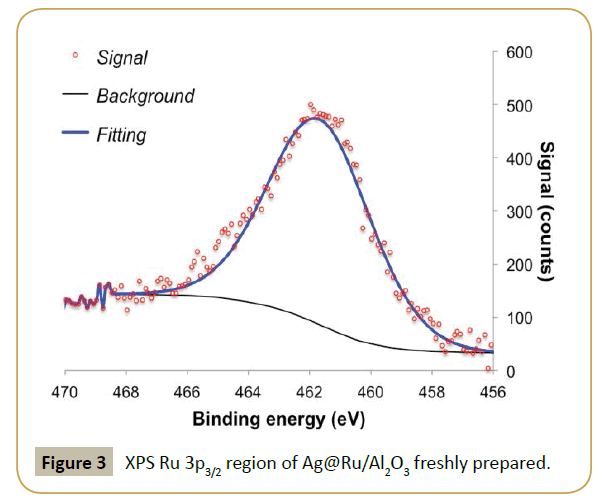
XPS analysis was performed to determine if the procedure was successful. Table 1 shows a clear drop in Ru/Al ratio in respect to the fresh parent catalyst and the appearance of a significant contribution of silver, i.e., high Ag/Al ratio. This result suggests that silver is deposited on top of the ruthenium particles, which diminishes the exposed ruthenium surface. Table 2, Figure 3 and 4 show the effect of adding silver to the ruthenium electronic structure. Silver addition led to a downward shift of ruthenium binding energy by ca. 0.5 eV. This shift suggests that silver electronic structure affected ruthenium electronic structure by donating electron density to it, as expected from tabulated electro negativities for Ru (2.20) and Ag (1.93). The observed binding energy of Ag 3d5/2 is assigned to silver in metallic state (Ag0 3d5/2=368.1-368.3 eV [31]).
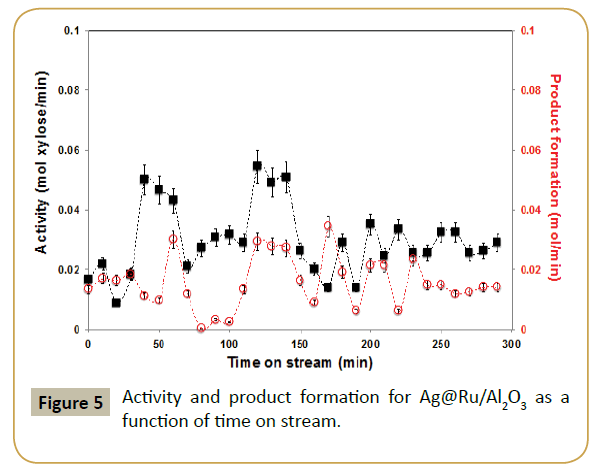
Figure 5 shows the effect in the catalysis when the parent ruthenium catalyst was modified with silver. Both conversion and selectivity were effected. Blank reaction with Ag/Al2O3 revealed that silver is not active in this reaction, at least with the conditions used. Hence, the drop in activity by addition of silver is easily justified as a geometric blocking of the active catalytic surface, i.e., decrease of ruthenium-exposed area where the reaction takes place. The product formation displays for the first half of the reaction time a very similar oscillatory behavior as with the parent system; even if in this case the oscillation periodicity has a lower frequency.
XPS analysis of the catalysts post-reaction showed an increase in Ru/Al ratio but almost the same Ag/Al ratio (Table 1). This suggests aggregation of silver atoms and formation of small silver nanoparticles on ruthenium surface. This is corroborated by antagonistic binding energy shift of Ru 3p3/2 and Ag 3d5/2 peaks after reaction (Table 2), suggesting a weaker interaction between the two metals. This is assumed to happen in the first part of the reaction since after 240 min on stream the oscillatory behavior of reaction profile decreased (Figure 5), resulting in an almost constant product formation of ca. 0.012 mol/min and substrate consumption at 0.028 mol/min. Since there were no other products detected in the Raman spectra, one suspect that the missing carbon content is on the catalyst in the form of coke or simply deposited carbon but further studies must be performed to reveal that.
The addition of silver was not dramatic but suggests that electronic modification of ruthenium holds the key to improve its performance, namely by promoting product desorption. Filling of the d-band of ruthenium softens both reagent and product adsorption, which should minimize competitive adsorption [32-36]. It is clear from post-reaction XPS analysis that very little amount of silver remains on ruthenium surface but that is sufficient to change the electronics of the metal.
Conclusion
In conclusion, a ruthenium ready-to-use in flow hydrogenation was prepared and successfully tested in D-xylose hydrogenation to xylitol with molecular hydrogen as reducing agent. The parent catalyst was efficaciously modified with silver metal, modifying modifying the surface and electronic structure of ruthenium The catalysts show a competitive effect between substrate adsorption and product desorption. Filling ruthenium d-band by silver doping seems to improve the process by softening the adsorption of the reagent and product. While there is a significant amount of work to be carried out to attain a catalyst with desired activity and product formation, such as establishing the best metal to use in the doping and its correct concentration, the presented work paved the way for the flow hydrogenation of D-xylose with ready-to- use catalysts that can be modified easily in situ.
Acknowledgement
The authors would like to thank Uppsala University and Stiftelsen Olle Engkvist Byggmästare (grant no. 2015/636) for the financial support.
References
- https://www.glyconutritionforlife.org/Science_of_Glyconutrients/Xylose.php
- Foster-Powell K, Holt SH, Brand-Miller JC (2002) International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76: 5-56.
- Edwardsson S, Birkhed D, Mejàre B (1977) Acid production from Lycasin®, maltitol, sorbitol and xylitol by oral streptococci and lactobacilli. Acta Odontol Scand 35: 257-263.
- Drucker DB, Verran J (1979) Changes in plaque pH in vitro by sweeteners. Arch Oral Biol 24: 965-970.
- https://www.researchandmarkets.com/reports/2846975/xylitol-a-global-market-overview
- Mikkola JP, Salmi T (2001) Three-phase catalytic hydrogenation of xylose to xylitol - prolonging the catalyst activity by means of on-line ultrasonic treatment. Catal Today 64: 271-277.
- van Gorp K, Boerman E, Cavenaghi CV, Berben PH, (1999) Catalytic hydrogenation of fine chemicals: sorbitol production. Catal Today 52: 349-361.
- Lee J, Kim YT, Huber GW (2014) Aqueous-phase hydrogenation and hydrodeoxygenation of biomass-derived oxygenates with bimetallic catalysts. Green Chem 16: 708-718.
- Yadav M, Mishra DK, Hwang JS (2012) Catalytic hydrogenation of xylose to xylitol using ruthenium catalyst on NiO modified TiO2 support. Appl Catal A Gen 425: 110-116.
- Hernandez-Mejia C, Gnanakumar ES, Olivos-Suarez A, Gascon J, Greer HF, et al. (2016) Ru/TiO2-catalysed hydrogenation of xylose: the role of the crystal structure of the support. Catal Sci Technol 6: 577-582.
- Mishra DK, Dabbawala AA, Hwang JS (2013) Ruthenium nanoparticles supported on zeolite Y as an efficient catalyst for selective hydrogenation of xylose to xylitol. J Molec Catal A: Chem 376: 63-70.
- Kwak BS, Lee BI, Kim TY, Kim JW, Lee SI (2006) WO 2006/093364 A1.
- Pham TN, Samikannu A, Rautio AR, Juhasz KL, Konya Z, et al. (2016) Catalytic hydrogenation of d-xylose over Ru decorated carbon foam catalyst in a SpinChem® rotating bed reactor. Top Catal 59: 1165-1177.
- Mallik M, Mandal RK (2008) Effect of variation of PVP/PVA weight ratio on the behaviour of nanocrystalline silver. Ind J Eng Mater Sci 15: 425-428.
- Larson PE, Kelly MA (1998) Surface charge neutralization of insulating samples in X-ray photoemission spectroscopy. J Vac Sci Technol A 16: 3483-3489.
- Strohmeier BR, Hercules DM (1984) Surface spectroscopic characterization of the interaction between zinc ions and γ-alumina. J Catal 86: 266-279.
- Ertl G, Hierl R, Knozinger H, Thiele N, Urbach HP (1980) XPS study of copper aluminate catalysts. Appl Surf Sci 5: 49-64.
- Berg C, Raaen S, Borg A, Andersen JN, Lundgren E, et al. (1993) Observation of a low-binding-energy peak in the 2p core-level photoemission from oxidized Al(111). Phys Rev B 47: 13063-13066.
- Fuggle JC, Madey TE, Steinkilberg M, Menzel D (1975) Photoelectron spectroscopic studies of adsorption of CO and oxygen on Ru (001). Surf Sci 52: 521-541.
- Fuggle JC, Kallne E, Watson LM, Fabian DJ (1977) Electronic structure of aluminum and aluminum-noble-metal alloys studied by soft-x-ray and x-ray photoelectron spectroscopies. Phys Rev B 16: 750-761.
- Folkesson B (1973) ESCA studies on the charge distribution in some dinitrogen complexes of rhenium, iridium, ruthenium, and osmium. Acta Chem Scand 27: 287-302.
- Koetz R, Lewrenz HJ, Stucki S (1983) XPS studies of oxygen evolution on Ru and RuO2 anodes. J Electrochem Soc 130: 825-829.
- Nyholm R, Martensson N (1980) Core level binding energies for the elements Zr-Te (Z=40-52). J Phys C 11: L279-L284.
- McEvoy AJ, Gissler W (1982) ESCA spectra and electronic properties of some ruthenium compounds. Phys Status Solidi A 69: K91-K96.
- Sarma DD, Rao CNR (1980) XPES studies of oxides of second- and third-row transition metals including rare earths. J Electron Spectroscopy Relat Phenom 20: 25-45.
- Zhang H, Fu Q, Yao Y, Zhang Z, Ma T, et al. (2008) Size-dependent surface reactions of Ag nanoparticles supported on highly oriented pyrolytic graphite. Langmuir 24: 10874-10878.
- Lopez-Salido I, Lin DC, Dietsche R, Bertram N, Kim YD (2006) Electronic and geometric properties of Au aanoparticles on highly ordered pyrolytic graphite (HOPG) studied using X-ray photoelectron spectroscopy (XPS) and scanning tunneling microscopy (STM). J Phys Chem B 110: 1128-1136.
- Ramstedt M, Franklyn P (2010) Difficulties in determining valence for Ag0 nanoparticles using XPS-characterization of nanoparticles inside poly (3-sulphopropyl methacrylate) brushes. Surf Interface Anal 42: 855-858.
- Paun C, Słowik G, Lewin E, Sá J (2016) Flow hydrogenation of p-nitrophenol with nano-Ag/Al2O3. RSC Adv 6: 87564-87568.
- Hammer B, Nørskov JK (1995) Why gold is the noblest of all the metals. Nature 376: 238-240.
- Cheng H, Lin W, Li X, Zhang C, Zhao F (2014) Selective hydrogenation of m-dinitrobenzeneto m-nitroaniline over Ru-SnOx/Al2O3 catalyst. Catalysts 4: 276-288.
- Sá J, Vinek H (2005) Catalytic hydrogenation of nitrates in water over a bimetallic catalyst. Appl Catal B: Environ 57: 247-256.
- Hammond JS, Gaarenstroom SW, Winograd N (1975) X-ray photoelectron spectroscopic studies of cadmium- and silver-oxygen surfaces. Anal Chem 47: 2193-2199.
- Johansson G, Hedman J, Berndtsson A, Klasson M, Nilsson R (1973) Calibration of electron spectra. J Electron Spectroscopy Relat Phenom 2: 295-317.
- Romand R, Roubin M, Deloume JP (1978) ESCA studies of some copper and silver selenides. J Electron Spectroscopy Relat Phenom 1: 229-242.
- Parry-Jones AC, Weightman P, Andrews PT (1979) The M4,5N4,5N4,5 Auger spectra of Ag, Cd, In and Sn. J Phys C 8: 1587-1600.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences